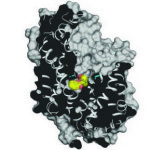
Synthetic radionuclides, such as the transuranic actinides plutonium, americium, and curium, present severe health threats as contaminants, and understanding the scope of the biochemical interactions involved in actinide uptake into cells is instrumental in managing human contamination. Recently, scientists have reported that an iron-binding protein called siderocalin can also bind and transport actinides into cells, a major advance in understanding the biological chemistry of radioactive metals. The research, which included X-ray crystallographic studies in the Berkeley Center for Structural Biology (Beamlines 5.0.1 and 5.0.2) at the Advanced Light Source (ALS), opens up new avenues of research into strategies for remedial action in the event of possible human exposure to nuclear contaminants. In addition, siderocalin, the protein studied, was selected as “Molecule of the Month” by the Protein Data Bank’s educational portal. Read more in the ALS Science Highlight.
Nature’s Microscopic Masonry: The First Steps in How Thin Protein Sheets Form Polyhedral Shells
Scientists for the first time have viewed how bacterial proteins self-assemble into thin sheets and begin to form the walls of the outer shell for nano-sized polyhedral compartments that function as specialized factories. Researchers in the Molecular Biophysics & Integrated Bioimaging Division determined the 3-D structure of the basic building block protein from crystallized samples in the Berkeley Center for Structural Biology at the Advanced Light Source. Their findings may eventually help improve drug delivery systems. Read more in the Berkeley Lab News Center.
Lab-Developed Beamline Tool Licensed
A diode beamstop technology for real time X-ray beam intensity measurement, developed by Diane Bryant and Simon Morton at the Berkeley Center for Structural Biology, was licensed to MiTeGen, which will commercialize a product to enhance X-ray beamlines used for pharma, materials, and biotech research. The Innovation and Partnerships Office managed the licensing process. Read more at IPO.
BCSB Helps Shed Light on Why Brain Responds to Dopamine and Serotonin
Scientists working at the Berkeley Center for Structural Biology in the Advanced Light Source (ALS) recently solved the crystallographic structures of several amine transporters in an effort to better understand why the human brain responds to chemicals like dopamine and serotonin. Their findings will help design drugs to treat neurological diseases and may also lead to a better understanding of how drug addiction can be managed. The work was led by HHMI Investigator Eric Gouaux of the Oregon Health & Science University. Read the ALS Science Highlight.
- « Previous Page
- 1
- …
- 10
- 11
- 12
Was this page useful?