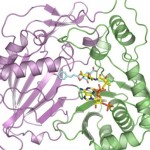
Recently, scientists from University of California, San Francisco, performed research at two national laboratories to determine protein structures that c0uld be the key to preventing Ebola infection. Alexander Kintzer and Robert Stroud, used two structural biology beamlines (5.0.2 and 8.3.1) at Berkeley Lab’s Advanced Light Source, to determine in atomic detail how a potential drug molecule fits into and blocks a channel in cell membranes that Ebola and related “filoviruses” need to infect victims’ cells. The study, published March 9 in Nature, marks an important step toward finding a cure for Ebola and other diseases that depend on the channel. Read more at the SLAC News Center.
New Way to Reduce Plant Lignin Could Lead to Cheaper Biofuels
Aymerick Eudes and Dominique Loqué of the Joint Bioenergy Institute (JBEI) led a study that shows for the first time that an enzyme can be tweaked to reduce lignin in plants. Their technique could help lower the cost of converting biomass into carbon-neutral fuels to power your car and other sustainably developed bio-products. The crystal structure of this enzyme was solved using data collected in the Berkeley Center for Structural Biology at the Advanced Light Source. Read more on the Berkeley Lab News Center.
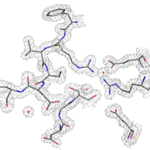
Improving Meningococcal Vaccines
Two recently licensed vaccines against bacterial meningitis contain a bacterial surface protein antigen known as Factor H binding protein (FHbp). The native form of this protein can have low thermal stability, which limits its potential use as an antigen in vaccines. After engineering a more stable Factor H binding protein antigen, scientists from UC San Francisco Benioff Children’s Hospital Oakland determined the structure of the stabilized vaccine with the help of protein crystallography at the Advanced Light Source (ALS) in the Berkeley Center for Structural Biology (Beamline 5.0.1). Read more in the ALS Science Brief.
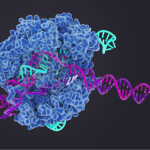
CRISPR/Cas9: Ready for Action
The CRISPR/Cas9 bacterial genomic editing system identifies and cleaves complementary target sequences in foreign DNA. CRISPR (clustered regularly interspaced short palindromic repeats)–associated (Cas) protein Cas9 begins its work by RNA-guided DNA unwinding to form an RNA-DNA hybrid and displacing a DNA strand inside the protein. Upon binding, Cas9 reorganizes into an R-loop complex that is necessary for it to perform its function. A recent article published in Science describes work done to uncover the structural basis of Cas9’s function.
A Key Step Toward Custom-Made Nanoscale Chemical Factories
Scientists have for the first time reengineered a building block of a geometric nanocompartment that occurs naturally in bacteria. The new design provides an entirely new functionality that greatly expands the potential for these compartments to serve as custom-made chemical factories. The work was led by Cheryl Kerfeld, who holds joint appointments with Berkeley Lab’s 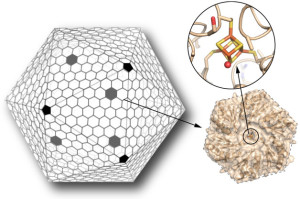 Molecular Biophysics and Integrated Bioimaging Division, UC Berkeley and the MSU-DOE Plant Research Laboratory at Michigan State University. Markus Sutter, a senior research associate in Kerfeld’s group at Berkeley Lab, collected the X-ray diffraction data used in this study in the Berkeley Center for Structural Biology at the Advanced Light Source. Read more at the Berkeley Lab News Center.
Molecular Biophysics and Integrated Bioimaging Division, UC Berkeley and the MSU-DOE Plant Research Laboratory at Michigan State University. Markus Sutter, a senior research associate in Kerfeld’s group at Berkeley Lab, collected the X-ray diffraction data used in this study in the Berkeley Center for Structural Biology at the Advanced Light Source. Read more at the Berkeley Lab News Center.
Was this page useful?