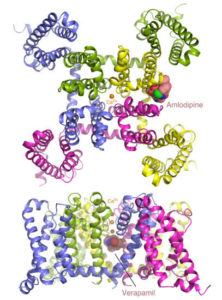
Calcium channel blockers are widely prescribed for heart and blood-vessel diseases. With the help of the high-intensity x-ray beams and remotely controlled robots at the Advanced Light Source (ALS), two groups of scientists from University of Washington have revealed, at atomic resolution, how two different classes of calcium channel blocker drugs produce their therapeutic effects. The researchers took advantage of the remotely controllable robot automounter available in the Berkeley Center for Structural Biology at the ALS to screen a large number of crystals for the best diffraction. The tunable x-ray source also allowed them to collect anomalous diffraction data at Beamlines 8.2.1 and 8.2.2 with bromine-incorporated drugs, which was critical for locating the drug molecules in the crystal. The results pave the way for optimizing these classic compounds for safer and more reliable pharmaceutical applications. Read the ALS Science Highlight.
Finding Diamonds in the Rough
New crystallography finding by JBEI and GLBRC benefits bioenergy industry
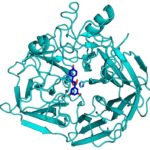 During the kraft process used to convert wood into wood pulp, the structural material lignin is partially converted into molecules like stilbene. Stilbenes are also naturally occurring in plants and some bacteria, and may play a role in plant pathogen resistance.
During the kraft process used to convert wood into wood pulp, the structural material lignin is partially converted into molecules like stilbene. Stilbenes are also naturally occurring in plants and some bacteria, and may play a role in plant pathogen resistance.
Currently, the deconstruction of plant biomass into cellulose and lignin is an expensive process. Lignin accounts for about 30 percent of plant cell wall carbon, and its conversion into chemicals or fuels could have a significant positive impact on the economics of processing lignocellulosic biomass. Enzymes capable of producing useful compounds from the breakdown of stilbenes and similar molecules could be employed for this. Collaborators from two of the Department of Energy Bioenergy Research Centers now have gained first-hand insight into how a stilbene cleaving oxygenase (SCO) carries out this unusual chemical reaction.
Understanding the Key to Henipavirus Infection
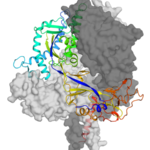
In 1994, a virus emerged in Hendra, Australia, causing respiratory and neurological diseases. It was transmissible from horses to humans, with a mortality rate of 57% in humans and 89% in horses. Since then 2 additional deadly species have emerged in Malaysia and Africa, with evidence of 19 more. The members of this Paramyxoviridae family infect host cells through the fusion protein, F, which is embedded in the viral particle membrane. The bulk of the F protein, the ectodomain, protrudes from the membrane’s surface and undergoes a dramatic refolding to merge the virus and host membranes. At the Advanced Light Source (ALS) Beamline 8.2.2 in the Berkeley Center for Structural Biology, researchers used macromolecular crystallography to study the structure of the Hendra F protein ectodomain in its prefusion form and gain insight into its function. Read the ALS Science Brief.
Bioscientists Validate Novel Protein Design Program
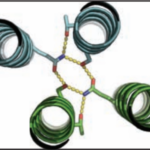
Over the course of billions of years, nature has evolved particular molecular structures that form the basis of life, such as those found in nucleic acids and proteins. Using the natural form as a springboard, University of Washington researchers have designed protein homo-oligomers, or identical interacting subunits, which can contain interchangeable hydrogen bonding modules for building different structures or functions. The team of researchers, led by David Baker at the University of Washington, included Jose Henrique Pereira, Banumathi Sankaran, and Peter Zwart of the Molecular Biophysics & Integrated Bioimaging Division (MBIB).
Visualizing Transporter Structure Creates Platform for Antidepressant Drug Design
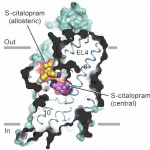
Researchers at Oregon Health and Sciences University’s Vollum Institute have revealed the molecular structure of the serotonin transporter (SERT), providing new insight into the mechanism of antidepressant action of two widely prescribed selective serotonin reuptake inhibitors (SSRIs) commonly used to treat depression. In their Nature paper, authors Jonathan Coleman, Evan Green, and Eric Gouaux describe their use of X-ray crystallography to capture images of human SERT structures. They collected data at the Beamline 5.0.2 in the Berkeley Center for Structural Biology and used the Phenix software suite to build models and refine the structures. The resulting structures show antidepressants citalopram and paroxetine lock SERT in an outward-open conformation, directly blocking serotonin binding. Visualizing this structure provides a blueprint for future drug design to treat anxiety and depression. This work was highlighted by Nature News and OHSU News.
- « Previous Page
- 1
- …
- 8
- 9
- 10
- 11
- 12
- Next Page »
Was this page useful?