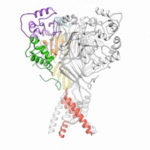
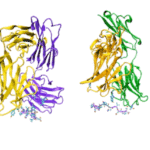
Data collected at the Berkeley Center for Structural Biology (BCSB) in the Advanced Light Source (ALS) has provided new structural insights into an antibody that protects against the bacterium that causes meningitis and sepis; a protein that unwinds quadruple DNA/RNA helixes; and an antibody targeting interleukin-2 that may provide a means of treating autoimmune disorders.
Biosciences’ Kenneth H. Downing Passes Away
The Berkeley Lab community mourns the loss of Kenneth H. Downing, who died August 2 at age 72. A senior scientist in the Molecular Biophysics and Integrated Bioimaging (MBIB) Division, Downing worked at the Lab for more than four decades. He passed away at home, surrounded by his family. A memorial service will be held on Saturday, September 1, at 11:00 a.m. at Lafayette Orinda Presbyterian Church, with a reception to follow.
Open and Shut: Pain Signals in Nerve Cells
Using protein crystallography at the Advanced Light Source (ALS), investigators at Oregon Health and Science University visualized an acid-sensing ion channel, ASIC1a, in it’s elusive closed state for the first time. An important cell membrane protein in the central nervous system, ASIC1a plays a role in sensing pain and in forming memories of fear. The researchers used protein crystals of ASIC1a channels purified in the closed state and compiled X-ray data from ALS Beamline 5.0.2 and the Advanced Photon Source at Argonne National Lab to build a model of the closed channel structure. Defining the closed structure enabled the researchers to generate a comprehensive molecular model of how ASIC1a toggles between the closed, open, and desensitized states.
Read more from the ALS.
Structures Reveal New Target for Malaria Vaccine
With the aid of protein crystallography at the Advanced Light Source (ALS), investigators at the National Institute of Allergy and Infectious Diseases (NIAID) and the Fred Hutchinson Cancer Research Center in Seattle led a study in which they isolated human-derived antibodies that protect against malaria and determined the antibodies’ site of attack. The team isolated the antibody, called CIS43, from the blood of a volunteer who had received an experimental vaccine made from whole, weakened malaria parasites (Plasmodium falciparum). The use of a whole-parasite vaccine allowed the isolation of antibodies against circumsporozoite protein (CSP), which covers the malaria parasite in its native conformation. The scientists co-crystallized CIS43 with various peptides from CSP. Protein crystallography at ALS Beamlines 5.0.1 and 5.0.2 revealed that CIS43 works by binding to a specific portion, or epitope, of CSP. This epitope occurs only once along the length of the surface protein and is conserved across 99.8 percent of all known strains of P. falciparum. The discovery paves the way for the development of a more effective and practical human vaccine for malaria, a disease responsible for half a million deaths worldwide each year.
Read more from the ALS.
ALS Welcomes Leda Insertion Device for Gemini Beamline
A critical component of the planned state-of-the-art macromolecular crystallography beamline Gemini has arrived at the Lab. The specially-designed in-vacuum undulator insertion device will yield higher photon flux compared to the Advanced Light Source’s superbend magnet and wiggler sources, bringing solutions to some of the most challenging projects in structural biology within reach.
Was this page useful?