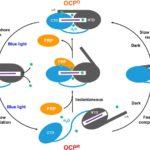
Researchers at Berkeley Lab and Michigan State University (MSU), led by Corie Ralston and Cheryl Kerfeld, performed X-ray footprinting mass spectrometry (XFMS) experiments at the Advanced Light Source (ALS) beamline 5.3.1, which revealed new mechanistic details of the key events in orange carotenoid protein (OCP) photoprotection. XFMS is ideally suited to probing conformational dynamics at the single residue level, providing both a spatial and temporal view of site-specific changes in the OCP and its interaction with the fluorescence recovery protein (FRP). The experiments showed that FRP provides an extended binding region that holds the OCP together and forces proximity of the two domains that accelerate relaxation of OCP to its native state.
Breakthrough in Membrane-Protein Design Settles Long-Standing Debate
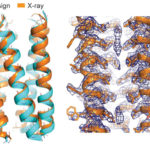
Membrane proteins that connect a cell’s inner workings with the outside world are essential for life and genetic mutations that affect their structural integrity or biological function are the cause of many diseases. Given their importance, researchers are interested in ascertaining the general physics principles underlying how membrane proteins fold and stabilize their molecular structures. Using high-resolution protein crystallography at the Advanced Light Source (ALS) Beamline 8.3.1, scientists from UCSF characterized designed membrane proteins to better understand the forces that stabilize these large, complex structures.
Read the ALS Science Highlight.
SIBYLS Sheds Light on Enzyme that Regulates Blood-clotting Factor
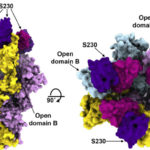
A group of researchers at Washington University School of Medicine have used the capabilities available at the Advanced Light Source’s SIBYLS beamline to gain insight into an enzyme that functions in blood clotting. ADAMTS13, which stands for a disintegrin and metalloproteinase with thrombospondin-1 repeats, member 13, is a multi-domain protease enzyme whose catalytic mechanism involves a metal. It is the only known protein to regulate the adhesive function of von Willebrand factor (VWF), a blood clotting protein involved in hemostasis.
BCSB Helps Elucidate Mechanism of Innate Immune Response
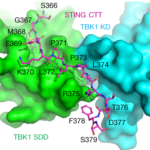
The crystallographic study of STING (stimulator of interferon genes), a transmembrane protein that plays a key role in innate immunity, in complex with TBK1 (serine/threonine-protein kinase), an enzyme that regulates the inflammatory response to foreign DNA, is extremely challenging due to weakly diffracting crystals. But thanks to the expertise of Berkeley Center for Structural Biology (BCSB) scientists, researchers from Texas A&M University (TAMU) were able to pinpoint the conserved motif of STING that mediates the recruitment and activation of TBK1. They published their results in Nature.
Antibody Uses Mimicry to Block SARS Coronavirus
To better understand how coronavirus antibodies work, a team of researchers at the University of Washington studied spike protein structures in complex with neutralizing antibody fragments isolated from SARS and MERS survivors. To visualize how the spike structures interact with the antibody fragments, they used a cryo-electron microscopy (cryo-EM) for the spikes, which are resistant to crystallization, and protein crystallography for the fragments. The high-resolution x-ray crystallography was performed at the Advanced Light Source (ALS) Beamline 5.0.1, part of the Berkeley Center for Structural Biology (BCSB).
- « Previous Page
- 1
- …
- 8
- 9
- 10
- 11
- 12
- …
- 17
- Next Page »
Was this page useful?