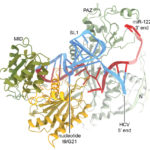
Viruses have evolved a wide variety of ways to exploit parts of their host cells to avoid detection and to grow. Researchers at the Scripps Research Institute and the Berkeley Center for Structural Biology are learning more about how hepatitis C works to deceive its host cells.
BCSB Determines Interactions of Potential Inhibitor with SARS-CoV-2 Protease
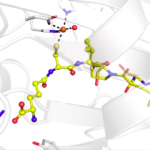
Researchers from the Baylor College of Medicine employed previously constructed DNA-encoded chemistry technology (DEC-tec) libraries to identify several candidate molecules that could inhibit the action of Mpro, the main protease of SARS-CoV-2. In a recent study, the researchers described CDD-1713, a new inhibitor to the enzyme Mpro that is involved in propagating the virus. The X-ray crystallographic data, which was collected by Banumathi Sankaran in the Molecular Biophysics and Integrated Bioimaging Division, allowed the researchers to determine that CDD-1713 inhibits the activity of Mpro by binding in the active site of this enzyme.
Enzyme Action Movie Shows How Nature Makes Penicillins
Scientists who specialize in studying the atom-by-atom choreography of enzymes have revealed new insights into the function of isopenicillin N synthase, an enzyme needed to produce some of the world’s most critical antibiotics.
Deconstructing the Infectious Machinery of the SARS-CoV-2 Virus
Scientists from three national laboratories who specialize in revealing the atomic structure of proteins collaborated to model the complex protein responsible for SARS-CoV-2 replication, revealing potential weak spots for drug development.
Q&A: Molecular Imaging Behind COVID-19 Breakthroughs
A team led by Paul Adams, director of Berkeley Lab’s Molecular Biophysics and Integrated Bioimaging Division, developed the Phenix software suite, now used around the world to automate key steps in the structural biology workflow. Adams spoke with the Strategic Communications about the software’s origins and how structural biologists leapt into action to combat the pandemic.
- 1
- 2
- 3
- …
- 5
- Next Page »
Was this page useful?