Paul Dabbar, Department of Energy’s Under Secretary for Science, visited Berkeley Lab on January 18. The visit focused namely on the Lab’s industry engagement programs. During the visit, Dabbar heard presentations by Mary Maxon, Associate Lab Director for Biosciences, and Blake Simmons, Division Director for Biological Systems and Engineering. Paul Adams, Division Director for Molecular Biophysics & Integrated Bioimaging welcomed Dabbar at the Advanced Light Source, and Todd Pray, Program Head of the Advanced Biofuels and Bioproducts Development Unit gave a tours of its facilities. The visit also included tours of the Integrative Genomics Building construction site and the Joint Bioenergy Institute.
Paul Dabbar, Department of Energy’s Under Secretary for Science, visited Berkeley Lab on January 18. The visit focused namely on the Lab’s industry engagement programs. During the visit, Dabbar heard presentations by Mary Maxon, Associate Lab Director for Biosciences, and Blake Simmons, Division Director for Biological Systems and Engineering. Paul Adams, Division Director for Molecular Biophysics & Integrated Bioimaging welcomed Dabbar at the Advanced Light Source, and Todd Pray, Program Head of the Advanced Biofuels and Bioproducts Development Unit gave a tours of its facilities. The visit also included tours of the Integrative Genomics Building construction site and the Joint Bioenergy Institute.
NIH Awards $6.5 Million for Augmenting Structural Biology Research Experience
The National Institutes of Health (NIH) has awarded $6.5 million to Berkeley Lab to integrate existing synchrotron structural biology resources to better serve researchers. The grant will establish a center based at the Lab’s Advanced Light Source (ALS) called ALS-ENABLE that will guide users through the most appropriate routes for answering their specific biological questions.
A Near-Atomic Resolution Map of a Key DNA Protein Complex
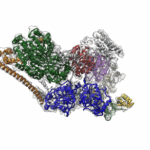
Molecular Biophysics and Integrated Bioimaging (MBIB) Division scientists led by Eva Nogales have resolved the 3-D structure of a critical human cellular protein complex involved in DNA transcription and repair at an unprecedented level of resolution. The complex, called transcription factor IIH (TFIIH), unzips the DNA double helix so that genes can be accessed and read. Malfunctions of the complex are associated with premature aging, cancer propensity, and a variety of other defects. One challenge with solving the structure of TFIIH has been that it exists in such minute amounts that it is difficult to produce and purify in large quantities. Moreover, once obtained, it may not form crystals suitable for X-ray diffraction. The researchers used cryo-electron microscopy (cryo-EM), a technique in which purified samples are flash-frozen at ultra cold temperatures, and which works even on very small quantities. “The fact that we resolved this protein structure from human cells makes this even more relevant to disease research,” said Nogales. Basil Greber, a postdoctoral fellow in Nogales’s lab, was first author on the study published in the journal Nature. Computational research scientist Pavel Afonine and MBIB Division Director Paul Adams also contributed to the project. Read more from the Berkeley Lab News Center.
NIH Awards $9.3M for Further Development of PHENIX Structural Biology Software
The National Institutes of Health (NIH) has awarded $9.3 million to Berkeley Lab to support ongoing development of PHENIX, a software suite for solving three-dimensional macromolecular structures. Officially launched in 2000, the project is a collaboration among researchers based at Berkeley Lab, Los Alamos National Laboratory, Cambridge University, and Duke University. “The impetus behind PHENIX is a desire to make the computational aspects of crystallography more automated, reducing human error and speeding solutions,” said PHENIX principal investigator Paul Adams, director of Berkeley Lab’s Molecular Biophysics and Integrated Bioimaging Division (MBIB). Read more from the Berkeley Lab News Center.
Could This Enzyme Help Turn Biofuel Waste into Something Useful?
 Researchers at the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) and Sandia National Laboratories working at the Joint BioEnergy Institute (JBEI) have resolved the protein structure of the enzyme LigM, which is utilized by the soil bacterium Sphingomonas to metabolize aryl compounds derived from lignin, the stiff, organic material that gives plants their structure. Their work is reported in this week’s Proceedings of the National Academy of Sciences. Read more in the Berkeley Lab News Center.
Researchers at the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) and Sandia National Laboratories working at the Joint BioEnergy Institute (JBEI) have resolved the protein structure of the enzyme LigM, which is utilized by the soil bacterium Sphingomonas to metabolize aryl compounds derived from lignin, the stiff, organic material that gives plants their structure. Their work is reported in this week’s Proceedings of the National Academy of Sciences. Read more in the Berkeley Lab News Center.
- « Previous Page
- 1
- …
- 5
- 6
- 7
- 8
- 9
- Next Page »
Was this page useful?