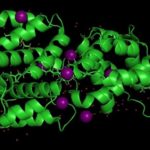
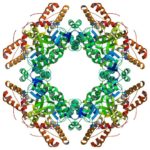
The Helical Carotenoid Protein 2 (HCP2) protein is an ancestor of proteins that are known to protect against damage caused by excess light exposure. Researchers in the laboratory of Cheryl Kerfeld, guest faculty in the Environmental Genomics & Systems Biology (EGSB) Division, are the first to structurally and biophysically analyze a protein from the HCP family. This HCP protein family was discovered recently by Kerfeld and the members of her lab, who are based in EGSB and at Michigan State University (MSU). To solve the molecular structure of HCP2, X-ray diffraction was measured at beam line 5.0.2 in the Berkeley Center for Structural Biology of the Advanced Light Source (ALS). The structure was refined using Phenix, a software suite for automated determination of molecular structures developed under the direction of Paul Adams, Molecular Biophysics and Integrated Bioimaging Division Director. Read more in the MSU-DOE Plant Research Laboratory news story.
Revealing the Shapes of Molecular Machines
Within each cell of the human body, thousands of molecular machines are at work. They transport nutrients and biochemicals into and out of our cells, build other tiny machines, and even move our cells around. To understand how these molecular machines work, scientists create three-dimensional pictures using electron cryomicroscopy (cryo-EM), catching these machines in different shapes that give insight into their function. Now researchers at Berkeley Lab and their international collaborators who write and distribute the Phenix software suite have developed a new set of computational tools for automated structure determination from cryo-EM data.
Researchers Identify Openings for Shuttering Virus Factories
A team led by Mary Estes of the Baylor College of Medicine used rotavirus as a model to study some of the proteins involved in making the cytoplasmic compartments in which many DNA and RNA virus pathogens replicate. Banumathi Sankaran, a research scientist in the Berkeley Center for Structural Biology (BSCB) at the Advanced Light Source, collected the X-ray data at the BCSB Beamline 5.0.1 that were used to solve the three-dimensional structures of nonstructural protein NSP2. Understanding the functions of proteins that make these compartments could offer an avenue for disrupting virus production. The team published their findings in Proceedings of the National Academy of Sciences.
Using Nature’s Blueprint for Sustainable Indigo Dyeing Process
Indigo has been prized since antiquity for its vibrancy and deep blue hue and, for more than a century, its unique properties have been leveraged to produce the popular textile blue denim. However, the dyeing process requires chemical steps that are environmentally damaging. A team of researchers in the Molecular Biophysics and Integrated Bioimaging (MBIB) and Biological Systems and Engineering (BSE) Divisions, at JBEI, and UC Berkeley have developed a promising sustainable indigo dyeing process that relies on genetically engineered bacteria, mimicking the natural biochemical protecting group strategy employed by the Japanese indigo plant Polygonum tinctorium.
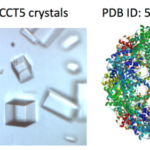
Crystallization Screen Created by Berkeley Lab Biosciences Scientists Reaches the Market
 X-ray crystallography has been the most successful technique used to solve macromolecular structures, contributing several thousand new entries to the Protein Data Bank (PDB) every year. The protein crystal is the critical starting point for X-ray data collection, and consequently, its properties are correlated with the quality of the data and the level of detail that can be extracted for a macromolecular structure. However, proteins require solutions of specific composition to form crystals for structure determination studies. These specifications are usually determined from exposing the protein to several different solutions in a crystallization screen.
X-ray crystallography has been the most successful technique used to solve macromolecular structures, contributing several thousand new entries to the Protein Data Bank (PDB) every year. The protein crystal is the critical starting point for X-ray data collection, and consequently, its properties are correlated with the quality of the data and the level of detail that can be extracted for a macromolecular structure. However, proteins require solutions of specific composition to form crystals for structure determination studies. These specifications are usually determined from exposing the protein to several different solutions in a crystallization screen.
A team of researchers in the Molecular Biophysics and Integrated Bioimaging (MBIB) Division led by Paul Adams and Jose Henrique Pereira have developed a new crystal screen, the Berkeley Screen, with 96 conditions proven to be highly effective at producing crystals for structural determination. The Berkeley Screen is now available to the wider crystallography community commercially.
- « Previous Page
- 1
- …
- 4
- 5
- 6
- 7
- 8
- 9
- Next Page »
Was this page useful?