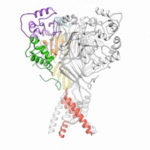
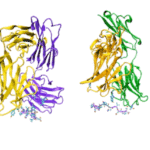
Data collected at the Berkeley Center for Structural Biology (BCSB) in the Advanced Light Source (ALS) has provided new structural insights into an antibody that protects against the bacterium that causes meningitis and sepis; a protein that unwinds quadruple DNA/RNA helixes; and an antibody targeting interleukin-2 that may provide a means of treating autoimmune disorders.
Infrared Beams Show Cell Types in a Different Light
Biosciences’ Cynthia McMurray and Mike Martin of the Advanced Light Source (ALS) are spearheading an effort to develop a noninvasive, label-free technique to probe living cells in their native environments to aid in biological and medical research. By shining highly focused infrared light—which doesn’t damage or otherwise alter the cells—they hope to be able to distinguish features within cells and identify individual cell types by their unique spectral signatures. McMurray, a senior scientist in Molecular Biophysics and Integrated Bioimaging (MBIB), and Martin, photon science operations group lead at the ALS, received a round of seed money earlier this year to support their effort, dubbed “spectral phenotyping.” An Aug. 8 news article in the journal Science highlighted their work and that of the larger Human Cell Atlas project that aims to provide “a unique ID card for each cell type,” as well as a 3D map of how cells form tissues, and new insights into disease.
Read more in the News Center.
Purdue Students Tour ALS
Corie Ralston, head of the Berkeley Center for Structural Biology and deputy director of the Molecular Biophysics and Integrated Bioimaging (MBIB) Division, and Mike Martin, Photon Science Operations Group Lead at the Advanced Light Source (ALS), led a tour of the ALS for a group of visiting biomedical engineering graduate students from Purdue University. Tammy Siemers, professional development and external relations specialist at Purdue University, arranges yearly ‘career immersion’ tours of companies and other places where graduate students might end up working one day. “The reason I chose ALS (and Lawrence Berkeley National Laboratory in general) is that I knew they were doing research in areas that our students would be interested in,“ said Siemers. “We had a great visit to ALS.”
The tours–which have taken place in Washington, DC, Boston, and Seattle previously–are important for the students to meet scientists and learn more about their options after graduate school. “I also like for students to gain networking skills and to learn about valuable resources and potential collaborations on this tour,” Siemers added. “ALS definitely has some valuable resources for research that we weren’t even aware of prior to this trip.”
Open and Shut: Pain Signals in Nerve Cells
Using protein crystallography at the Advanced Light Source (ALS), investigators at Oregon Health and Science University visualized an acid-sensing ion channel, ASIC1a, in it’s elusive closed state for the first time. An important cell membrane protein in the central nervous system, ASIC1a plays a role in sensing pain and in forming memories of fear. The researchers used protein crystals of ASIC1a channels purified in the closed state and compiled X-ray data from ALS Beamline 5.0.2 and the Advanced Photon Source at Argonne National Lab to build a model of the closed channel structure. Defining the closed structure enabled the researchers to generate a comprehensive molecular model of how ASIC1a toggles between the closed, open, and desensitized states.
Read more from the ALS.
Structures Reveal New Target for Malaria Vaccine
With the aid of protein crystallography at the Advanced Light Source (ALS), investigators at the National Institute of Allergy and Infectious Diseases (NIAID) and the Fred Hutchinson Cancer Research Center in Seattle led a study in which they isolated human-derived antibodies that protect against malaria and determined the antibodies’ site of attack. The team isolated the antibody, called CIS43, from the blood of a volunteer who had received an experimental vaccine made from whole, weakened malaria parasites (Plasmodium falciparum). The use of a whole-parasite vaccine allowed the isolation of antibodies against circumsporozoite protein (CSP), which covers the malaria parasite in its native conformation. The scientists co-crystallized CIS43 with various peptides from CSP. Protein crystallography at ALS Beamlines 5.0.1 and 5.0.2 revealed that CIS43 works by binding to a specific portion, or epitope, of CSP. This epitope occurs only once along the length of the surface protein and is conserved across 99.8 percent of all known strains of P. falciparum. The discovery paves the way for the development of a more effective and practical human vaccine for malaria, a disease responsible for half a million deaths worldwide each year.
Read more from the ALS.
- « Previous Page
- 1
- …
- 12
- 13
- 14
- 15
- 16
- …
- 19
- Next Page »
Was this page useful?