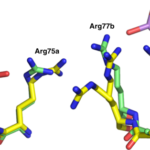
A newly-discovered arsenic-containing compound produced by a soil bacterium shows promise as a broad-spectrum antibiotic. In a paper published in the Nature journal Communications Biology, an international team of researchers demonstrated that arsinothricin (AST) is effective against many types of gram-negative and gram-positive bacteria. The effort was led by Barry Rosen of the Florida International University College of Medicine and Masafumi Yoshinaga of the National Agriculture and Food Research Organization (NARO) in Japan. Banumathi Sankaran, a research scientist in the Berkeley Center for Structural Biology (BSCB) at the Advanced Light Source (ALS), was an author on the paper.
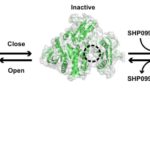
Locking Protein Structure to Close the Door on Cancer
Mutations in the proteins that regulate cellular processes such as growth, division, and death are often linked to cancer and other diseases. The proper function of one of these proteins, SHP2, depends on maintaining equilibrium in a structural tug-of-war between an open (active) and a closed (inactive) arrangement. A team of researchers from Brandeis University performed X-ray crystallography at Advanced Light Source (ALS) Beamlines 8.2.1 and 8.2.2—part of the Berkeley Center for Structural Biology—to elucidate the structures of healthy and mutated forms of SHP2 and the dynamic interchange between their open and closed conformations, as well as how SHP2 interacts with certain cancer drugs.
Read more in this ALS Science Highlight.
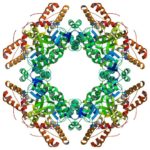
Researchers Identify Openings for Shuttering Virus Factories
A team led by Mary Estes of the Baylor College of Medicine used rotavirus as a model to study some of the proteins involved in making the cytoplasmic compartments in which many DNA and RNA virus pathogens replicate. Banumathi Sankaran, a research scientist in the Berkeley Center for Structural Biology (BSCB) at the Advanced Light Source, collected the X-ray data at the BCSB Beamline 5.0.1 that were used to solve the three-dimensional structures of nonstructural protein NSP2. Understanding the functions of proteins that make these compartments could offer an avenue for disrupting virus production. The team published their findings in Proceedings of the National Academy of Sciences.
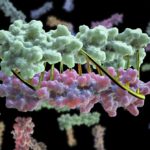
Programming Proteins to Pair Perfectly
Bioscientists at the Advanced Light Source (ALS) at Berkeley Lab lent their expertise to a project led by scientists at the University of Washington to design proteins in the lab that zip together like DNA. The technique could enable the design of protein nanomachines to help diagnose and treat disease, allow for more precise engineering of cells, and perform a variety of other tasks.
Toward a Blueprint for Anti-influenza Drugs
An international team led by researchers at UCSF used protein crystallography at the Advanced Light Source (ALS) beamline 8.3.1 to obtain structures of several influenza antiviral drug molecules bound to their proton-channel targets in both open and closed conformations. These complexes provide the first high-resolution views of how the drugs interact with and disrupt the water-molecule networks lining the M2 transmembrane channel. The structures provide an atomic-level blueprint from which to design more effective anti-influenza drugs that can overcome growing drug resistance. ALS beamline 8.3.1 is operated by James Holton, MBIB beamline scientist and associate adjunct professor at UCSF.
Read more in the ALS Science Highlight.
- « Previous Page
- 1
- …
- 9
- 10
- 11
- 12
- 13
- …
- 17
- Next Page »
Was this page useful?