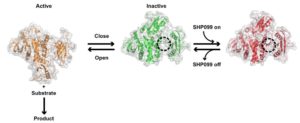
Mutations in the proteins that regulate cellular processes such as growth, division, and death are often linked to cancer and other diseases. The proper function of one of these proteins, SHP2, depends on maintaining equilibrium in a structural tug-of-war between an open (active) and a closed (inactive) arrangement. A team of researchers from Brandeis University performed X-ray crystallography at Advanced Light Source (ALS) Beamlines 8.2.1 and 8.2.2—part of the Berkeley Center for Structural Biology—to elucidate the structures of healthy and mutated forms of SHP2 and the dynamic interchange between their open and closed conformations, as well as how SHP2 interacts with certain cancer drugs.
Read more in this ALS Science Highlight.



