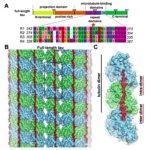
Researchers at Berkeley Lab and UC Berkeley have combined cutting-edge cryo-electron microscopy (cryo-EM) with computational molecular modeling to produce a near atomic-resolution model of the interaction between microtubules—crucial components of eukaryotic cell ultrastructure—and microtubule-associated proteins called tau. The model provides insight into how tau stabilizes microtubules, and what makes it dissociate to form tau aggregates, or “tangles,” in some neurological diseases—including Alzheimer’s disease—generally referred to as tauopathies.
Cryo-EM Reveals Structure of Human Telomerase
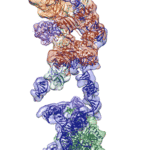
Regulation of the enzyme telomerase has been implicated in cancer, other human diseases, and aging, but progress towards therapeutic manipulation of telomerase has been hampered by the lack of high-resolution structural data. Now, researchers in senior faculty scientist Eva Nogales’ lab in Molecular Biophysics and Integrated Bioimaging (MBIB), in collaboration with UC Berkeley professor of Biochemistry, Biophysics and Structural Biology Kathleen Collins, have published a paper in Nature describing the 3-D molecular structure of the human telomerase enzyme.
Silencing Is Golden: Scientists Image Molecules Vital for Gene Regulation
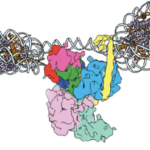
In two new studies, a team of researchers led by Eva Nogales, senior faculty scientist in the Molecular Biophysics and Integrated Bioimaging (MBIB) Division, has gained insight into the structure and function of molecules that act at the genetic level to give rise to different types of cells.
Dernburg and Nogales Named Fellows of the American Society for Cell Biology
Biosciences’ Abby Dernburg and Eva Nogales–both of whom are also UC Berkeley professors and HHMI Investigators–have been selected as 2017 Fellows of the American Society for Cell Biology (ASCB). Election as a Fellow is an honor bestowed upon ASCB members by their peers. The award is a lifetime recognition of meritorious efforts to advance cell biology and/or its applications, work in service to the Society, and ongoing loyalty to ASCB.
Detailed View of Immune Proteins Could Lead to New Pathogen-Defense Strategies
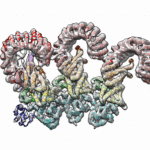
Berkeley Lab and UC Berkeley biologists used cryo-electron microscopy (cryo-EM) to resolve the structure of a protein complex, called an inflammasome, that is assembled within cells in response to incursion by a pathogen and functions as a beacon to summon immune system support. Inflammasome assembly is initiated when a protein called NAIP5 latches onto a flagellin molecule—a piece of the whiplike tail used by bacteria to propel themselves. Then several copies of another protein, NLRC4, join in to form the ring-shaped protein cluster. The researchers, led by Eva Nogales in Biosciences’ Molecular Biophysics and Integrated Bioimaging (MBIB) Division and Russell Vance in the Department of Molecular & Cell Biology at UCB, found that flagellin is in contact with six different parts of NAIP5. They further demonstrated that the multiple contact sites help prevent bacteria with minor mutations from eluding detection. The study was published in the journal Science. Read more in the Berkeley Lab News Center.
Was this page useful?