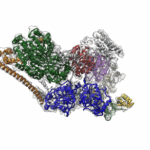
Molecular Biophysics and Integrated Bioimaging (MBIB) Division scientists led by Eva Nogales have resolved the 3-D structure of a critical human cellular protein complex involved in DNA transcription and repair at an unprecedented level of resolution. The complex, called transcription factor IIH (TFIIH), unzips the DNA double helix so that genes can be accessed and read. Malfunctions of the complex are associated with premature aging, cancer propensity, and a variety of other defects. One challenge with solving the structure of TFIIH has been that it exists in such minute amounts that it is difficult to produce and purify in large quantities. Moreover, once obtained, it may not form crystals suitable for X-ray diffraction. The researchers used cryo-electron microscopy (cryo-EM), a technique in which purified samples are flash-frozen at ultra cold temperatures, and which works even on very small quantities. “The fact that we resolved this protein structure from human cells makes this even more relevant to disease research,” said Nogales. Basil Greber, a postdoctoral fellow in Nogales’s lab, was first author on the study published in the journal Nature. Computational research scientist Pavel Afonine and MBIB Division Director Paul Adams also contributed to the project. Read more from the Berkeley Lab News Center.
Cryo-Electron Microscopy Achieves Unprecedented Resolution Using New Computational Methods
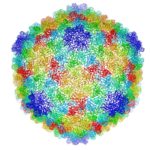
Cryo-electron microscopy is a critical tool used to advance biochemical knowledge. Now Pavel Afonine, research scientist, and Molecular Biophysics and Integrated Bioimaging Division Director Paul Adams have extended cryo-EM’s impact further by developing a new computational algorithm that was instrumental in constructing a 3-D atomic-scale model of bacteriophage P22 for the first time. Read more in the Berkeley Lab News Center.
The Strings That Bind Us: Cytofilaments Connect Cell Nucleus to Extracellular Microenvironment
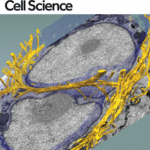
New images of structural fibers inside a cell appear in a study featured on the cover of the Journal of Cell Science special issue on 3D Cell Biology, published this month. The images, obtained by scientists in the Biosciences Area, show thread-like cytofilaments reaching into and traversing a human breast cell’s chromatin-packed nucleus. 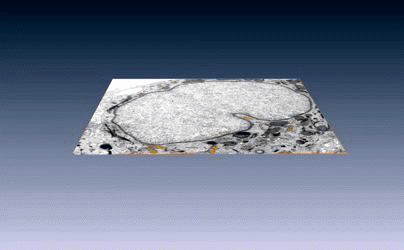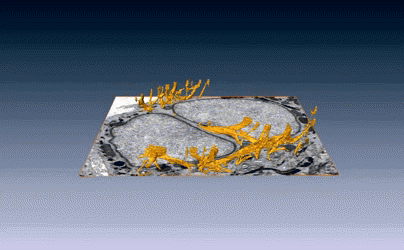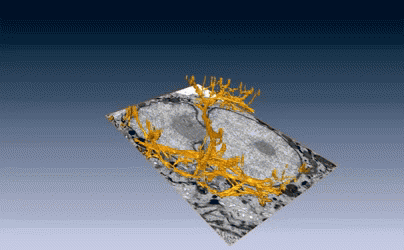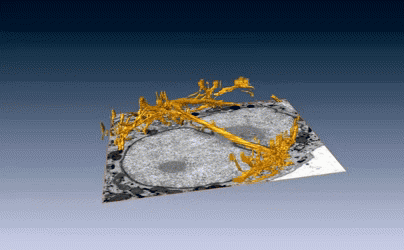 It provides the first visual evidence of a physical link by which genes can receive mechanical cues from its microenvironment.
It provides the first visual evidence of a physical link by which genes can receive mechanical cues from its microenvironment.
The work leading up to the images began in the early 1980s when Biological Systems & Engineering’s Mina Bissell proposed the idea that gene expression and cell fate were dependent on their physical surroundings called extracellular matrix. The images were captured by Manfred Auer, staff scientist, and Ke Xu, faculty scientist, both in the Molecular Biophysics & Integrated Bioimaging Division. Read more at the Berkeley Lab News Center.
Workshop Focuses in on Electron Microscopy
An all-day workshop highlighting the Berkeley Lab’s capabilities in electron microscopy was held on Tuesday, October 11. Organized by Paul Adams (Biosciences Area), Peter Denes (Advanced Light Source) and Andy Minor (National Center for Electron Microscopy), the workshop highlighted recent advances in imaging a broad range of materials and biological samples at atomic, or near-atomic scales. In addition, it made evident the many opportunities that could come from integrating capabilities across the Laboratory. Paul Adams, Director of the Molecular Biophysics & Integrated Bioimaging Division, noted that the recent revolution in electron microscopy for biosciences has opened up many new avenues of research and exciting synergies with non-biosciences programs at the Lab. Read more in Glenn Roberts’ Science Short on the Berkeley Lab News Center.
New Technologies Fuel Cryo-EM’s Renaissance
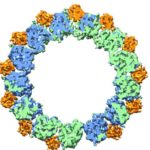
In a pair of breakthrough Nature papers published recently, researchers in Eva Nogales’ Lab at UC Berkeley and Berkeley Lab (Molecular Biophysics & Integrated Bioimaging Division) mapped two important protein functions in unprecedented detail: the role of TFIID, effectively improving our understanding of how our molecular machinery identifies the right DNA to copy; and how proteins unzip double-stranded DNA, which gives us insights into the first-key steps in gene activation.
These papers are representative of the renaissance currently under way in the cryo-electron microscopy (cryo-EM) field—driven primarily by the rise of cutting-edge electron detector cameras, sophisticated image processing software and access to NERSC supercomputing resources. Read the full story, written by Linda Vu of NERSC.
- « Previous Page
- 1
- …
- 4
- 5
- 6
- 7
- Next Page »
Was this page useful?