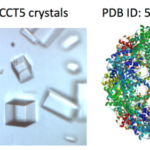
X-ray crystallography has been the most successful technique used to solve macromolecular structures, contributing several thousand new entries to the Protein Data Bank (PDB) every year. The protein crystal is the critical starting point for X-ray data collection, and consequently, its properties are correlated with the quality of the data and the level of detail that can be extracted for a macromolecular structure. However, proteins require solutions of specific composition to form crystals for structure determination studies. These specifications are usually determined from exposing the protein to several different solutions in a crystallization screen.
X-ray crystallography has been the most successful technique used to solve macromolecular structures, contributing several thousand new entries to the Protein Data Bank (PDB) every year. The protein crystal is the critical starting point for X-ray data collection, and consequently, its properties are correlated with the quality of the data and the level of detail that can be extracted for a macromolecular structure. However, proteins require solutions of specific composition to form crystals for structure determination studies. These specifications are usually determined from exposing the protein to several different solutions in a crystallization screen.
A team of researchers in the Molecular Biophysics and Integrated Bioimaging (MBIB) Division led by Paul Adams and Jose Henrique Pereira have developed a new crystal screen, the Berkeley Screen, with 96 conditions proven to be highly effective at producing crystals for structural determination. The Berkeley Screen is now available to the wider crystallography community commercially.