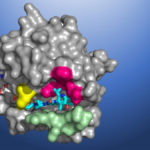
Seeking to develop a direct inhibitor of a mutant protein caused by errors in the KRAS gene, researchers at Amgen conducted X-ray crystallography of KRAS(G12C) proteins using the Berkeley Center for Structural Biology (BCSB) beamlines at the Advanced Light Source (ALS). The high-resolution structural maps generated from the data acquired revealed a small pocket on the molecule. Now, an investigational cancer drug that binds in this pocket will be evaluated in phase 2 clinical trials.
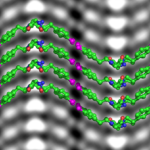
Cryo-EM Adapted to Visualize Atomic Structure of Synthetic Soft Material
Berkeley Lab scientists have demonstrated for the first time that cryogenic electron microscopy (cryo-EM), a Nobel Prize-winning technique originally designed to image proteins in solution, can be adapted to image atomic changes in a synthetic soft material.
BCSB Helps Validate Citizen-scientist Designed Protein Structures
Berkeley Center for Structural Biology (BCSB) beamlines 5.0.2, 8.2.1, and 8.2.2 at the Advanced Light Source (ALS) were used to experimentally verify de novo protein structures designed by citizen scientists playing the computer game Foldit.
A Frog Worth Kissing: Natural Defense Against Red Tide Toxin Found in Bullfrogs
A team led by Berkeley Lab faculty biochemist Daniel Minor has discovered how a protein produced by bullfrogs binds to and inhibits the action of saxitoxin, the deadly neurotoxin made by cyanobacteria and dinoflagellates that causes paralytic shellfish poisoning. The findings, published this week in Science Advances, could lead to the first-ever antidote for the compound, which blocks nerve signaling in animal muscles, causing death by asphyxiation when consumed in sufficient quantities.
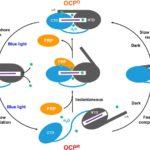
X-ray Footprinting Reveals Molecular Basis of Orange Carotenoid Protein Photoprotection
Researchers at Berkeley Lab and Michigan State University (MSU), led by Corie Ralston and Cheryl Kerfeld, performed X-ray footprinting mass spectrometry (XFMS) experiments at the Advanced Light Source (ALS) beamline 5.3.1, which revealed new mechanistic details of the key events in orange carotenoid protein (OCP) photoprotection. XFMS is ideally suited to probing conformational dynamics at the single residue level, providing both a spatial and temporal view of site-specific changes in the OCP and its interaction with the fluorescence recovery protein (FRP). The experiments showed that FRP provides an extended binding region that holds the OCP together and forces proximity of the two domains that accelerate relaxation of OCP to its native state.
- « Previous Page
- 1
- …
- 9
- 10
- 11
- 12
- 13
- …
- 19
- Next Page »
Was this page useful?