To perform their functions, such as transcribing the information contained in our genes or to repairing the dozens of strand breaks that occur daily, our enzymes must be able to directly access the DNA within our cells’ nuclei. However, this access is limited because the DNA strands are often tightly coiled around proteins like threads around spools. The strands must be carefully unwound by various enzymes, including one called TIP60, to make them accessible for duplication and gene expression. When TIP60 malfunctions, the enzymes that repair breaks in the DNA strands can’t access them, and significant cellular damage can occur.
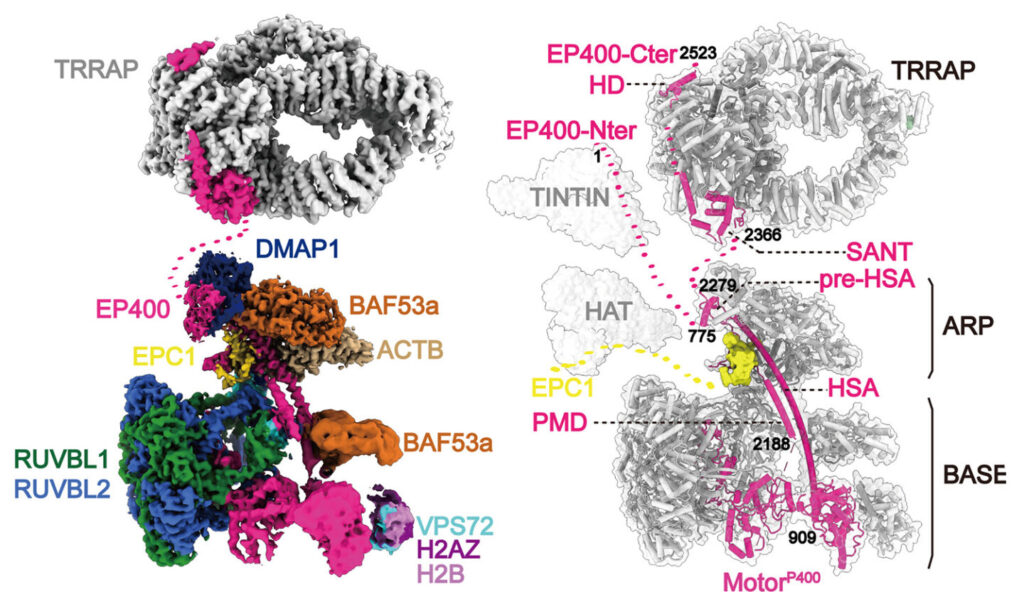
Using specialized equipment, including high-resolution cryo-electron microscopy, researchers from Berkeley Lab, UC Berkeley, the Institute for Systems Biology, and Université Laval were able to study the structure of this complex, which is made up of 17 proteins, and the interactions between its components. Knowing the detailed structure and behavior of TIP60 could provide insight into different diseases where the protein complex plays a role, such as Alzheimer’s and various cancers, and help develop new targeted therapies. The work was reported in the journal Science.
“This collaborative work brings together structure and functional assays in a powerful way to inform us on how this complex macromolecular assembly carries out its job to regulate the reading of our genome,” said Eva Nogales, a senior faculty scientist in Molecular Biophysics and Integrated Bioimaging (MBIB), UC Berkeley professor, and Howard Hughes Medical Institute investigator. To shed light on the structure of TIP60, Nogales and her team purified and studied samples prepared by the Côté group at Université Laval. “The structure of the human TIP60 reveals how evolution has led to the merging of two distinct molecular functions into a single complex, readjusting the way structural modules come together to fit its dual functionality,” she added.



