Great progress has recently been made in understanding the cascade of molecular signals that trigger cell replication, and compounds that block these signals when they run amok are of great interest as potential cancer treatments. While such drugs have shown efficacy in the clinic, resistance mechanisms that reactivate the signaling pathway have emerged. Thus, it is vitally important to gain a molecular-level understanding of the various ways in which signaling proteins interact with each other.
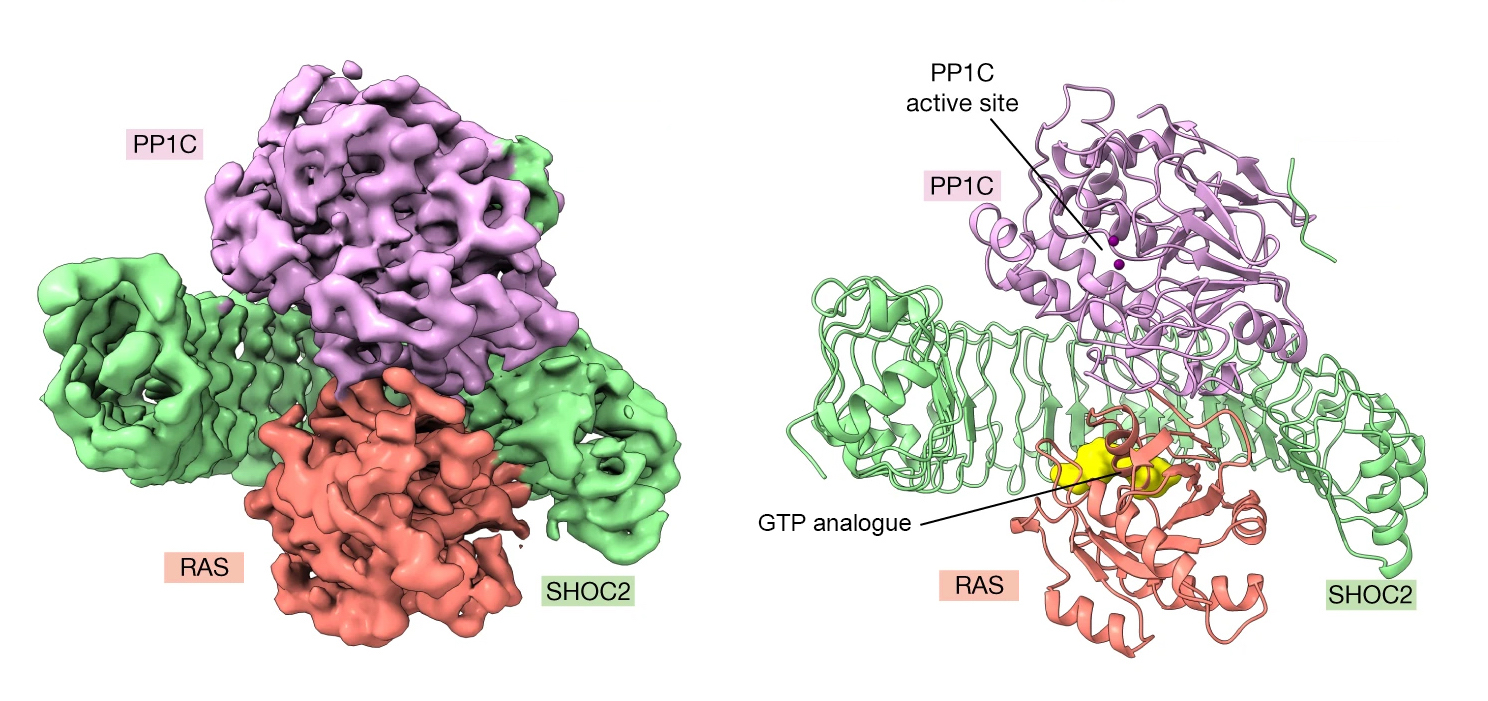
To that end, a research group from Genentech used a suite of biochemical and structural methods to learn how an assembly of three proteins works together to transmit signals for cell division. The latter included small-angle x-ray scattering (SAXS) on the SIBYLS beamline (12.3.1) at the Advanced Light Source (ALS). SAXS captures snapshots of large, flexible protein complexes suspended in solution, enabling the researchers to visualize dynamic aspects of complex formation. At the beamline, samples were also analyzed using size exclusion chromatography (SEC) and multi-angle light scattering (MALS). This high-throughput SEC-SAXS-MALS capability was important for validating various complex assemblies prior to cryo-EM studies.
The work reveals new targets for the development of drugs that fight certain types of cancer, including lung, colorectal, and pancreatic.



