
The National Institutes of Health (NIH) recently awarded more than $40 million, distributed over five years, for a project that aims to compare two positron emission tomography (PET) imaging agents widely used to visualize misfolded tau protein—which, along with amyloid plaques, is a hallmark of Alzheimer disease (AD)—in living brains. The effort will be led by co-principal investigators Suzanne Baker, a computational staff scientist in the Molecular Biophysics and Integrated Bioimaging (MBIB) Division, and Tharick Pascoal, an assistant professor in the department of psychiatry at the University of Pittsburgh (Pitt).
Normally, tau forms part of tube-shaped structures that give neurons internal stability and transport nutrients, among other things. But instead of assembling in an orderly way, abnormal tau gloms together in messy jumbles, which interfere with neuronal function. The amount and distribution of these neurofibrillary tangles in the brain is correlated with cognitive impairment, making tau a useful biomarker for AD progression and a promising target for therapeutic interventions. It is therefore critically important to be able to accurately quantify tau and measure change over time.
To see where in the brain tau has accumulated and how much is there, researchers use mildly radioactive compounds called tracers that are designed to bind to tau and make it visible on a PET scan. However, in addition to binding to their target, tracers may also bind to other proteins that share some similar features. Baker, Pascoal, and their collaborators want to definitively characterize the different binding affinities of two popular tau tracers, [18F]Flortaucipir and [18F]MK-6240, for tau aggregates, as well as their off-target signals.
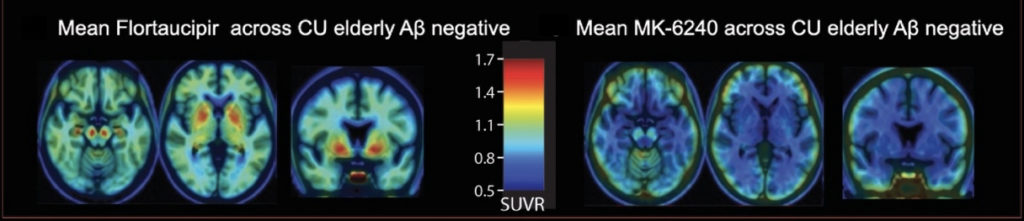
By comparing [18F]Flortaucipir and [18F]MK-6240 head-to-head, the researchers are hoping to better understand the strengths and weaknesses of each tracer. Their ultimate goal is to derive transformation parameters to convert tau PET tracers to a common scale. “If you’re designing a specific kind of study, maybe one tracer is better for a certain stage of the disease and another tracer better for another stage,” said Baker. “It’s also really important to be able to compare, and possibly combine, data from the two different tracers,” she added.
Read more about the grant in the the Pitt press release



