A team of UC Berkeley and Berkeley Lab researchers used X-ray crystallography performed at the Advanced Light Source (ALS) to determine the atomic structure of ORF8, a protein secreted by the SARS-CoV-2 virus that is thought to help the pathogen evade and dampen response from human immune cells.
James Hurley, a UC Berkeley professor and former faculty scientist in the Molecular Biophysics and Integrated Bioimaging (MBIB) Division, worked with Marc Allaire, a biophysicist and crystallography expert at the Berkeley Center for Structural Biology (BCSB), located at the ALS. The ALS had received special funding from the CARES Act to remain operational for COVID-19 investigations.
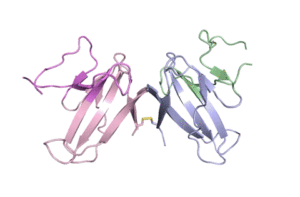
“We built an atomic model of ORF8, and it highlighted two unique regions: one that is only present in SARS-CoV-2 and its immediate bat ancestor, and one that is absent from any other coronavirus,” said Hurley. “These regions stabilize the protein … and create new intermolecular interfaces. We, and others in the research community, believe these interfaces are involved in reactions that somehow make SARS-CoV-2 more pathogenic than the strains it evolved from.”
The team opted to share their findings with the scientific community via an open-access data bank prior to publication in a peer-reviewed journal, laying the groundwork for other groups to study ORF8 in more detail. Their structure map has now been published in the journal PNAS.
Read more in the Berkeley Lab News Center.