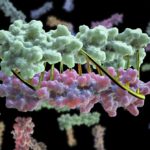
A group of researchers at Washington University School of Medicine have used the capabilities available at the Advanced Light Source’s SIBYLS beamline to gain insight into an enzyme that functions in blood clotting. ADAMTS13, which stands for a disintegrin and metalloproteinase with thrombospondin-1 repeats, member 13, is a multi-domain protease enzyme whose catalytic mechanism involves a metal. It is the only known protein to regulate the adhesive function of von Willebrand factor (VWF), a blood clotting protein involved in hemostasis.
Programming Proteins to Pair Perfectly
Bioscientists at the Advanced Light Source (ALS) at Berkeley Lab lent their expertise to a project led by scientists at the University of Washington to design proteins in the lab that zip together like DNA. The technique could enable the design of protein nanomachines to help diagnose and treat disease, allow for more precise engineering of cells, and perform a variety of other tasks.
Berkeley Lab Bioscientists Participate in CASP13
CASP is the Critical Assessment of Protein Structure Predictions, a biannual “competition” to determine which prediction algorithm generates the most accurate model. There are several categories in which models will be assessed, including accuracy, topology, and biological relevance. The SIBYLS beamline is participating to provide small-angle X-ray scattering (SAXS) data for––and judging for the first time––the “data-assisted” category. This CASP competition should lead to improvement in predicting protein-protein interfaces and complex structures.
NIH Awards $6.5 Million for Augmenting Structural Biology Research Experience
The National Institutes of Health (NIH) has awarded $6.5 million to Berkeley Lab to integrate existing synchrotron structural biology resources to better serve researchers. The grant will establish a center based at the Lab’s Advanced Light Source (ALS) called ALS-ENABLE that will guide users through the most appropriate routes for answering their specific biological questions.
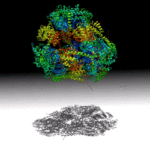
A Hollow Pyramid Unlocks Principles of Protein Architecture
Researchers at UCLA have designed a hollow, pyramid-shaped protein with a controllable cavity size that may aid the capture and release of smaller compounds. The tools used in this work, including small angle X-ray scattering techniques at the SIBYLS beamline in the Advanced Light Source (ALS), will help analyze and optimize designed-protein assemblies and understand their behavior in solution. Read more in the ALS Science Brief.
Was this page useful?