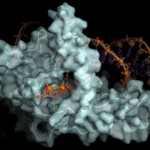
Using a combination of crystallographic, biochemical, and genetic analyses, Berkeley Lab researchers have shown that the actions of FEN1, an enzyme involved in DNA replication and repair, are guided by electrostatic forces known as phosphate steering. Susan Tsutakawa and John Tainer in the Biosciences Area’s Molecular Biophysics and Integrated Bioimaging (MBIB) Division were the lead and corresponding authors, respectively, on the report published this week in Nature Communications. The work reveals key details of this previously unknown mechanism controlling the specificity of FEN1 in healthy cells and provides new directions for cancer treatment research. Read more from the Berkeley Lab News Center.
Unexpected Key Role for Unfolded Protein Regions in DNA Break Repair Defined
Structurally Integrated Biology for Life Sciences (SIBYLS) beamline researchers, led by research scientist Michal Hammel of the Molecular Biophysics & Integrated Bioimaging (MBIB) Division, used X-ray scattering to define an unexpected key role for unfolded protein regions in DNA break repair to allow regulation plus access to DNA ends. The concept of DNA break repair as a flexibly-linked dynamic complex, as opposed to a linear pathway, suggests new approaches to targeting DNA repair for selectively killing cancer cells due to their high levels of DNA instability. Their findings were published in a recent cover article of the Journal of Biological Chemistry.
The SIBYLS beamline of the Advanced Light Source at Berkeley Lab, directed by MBIB’s senior scientist John Tainer, is optimized for both small-angle X-ray scattering (SAXS) and macromolecular crystallography (MX), making it unique among the world’s mostly SAXS or MX dedicated beamlines.
Was this page useful?