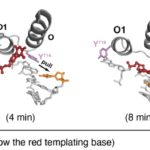
Every time our cells divide, the DNA inside must be copied accurately to avoid mistakes that could be harmful to our health. Known as DNA synthesis, the precise sequence of steps has until now only been hypothesized. In a recent study, timestamps have been added to step-by-step snapshots, revealing a switch-up between two of the steps that, if replicated in additional studies, would upend our current assumptions of the process.
BCSB Determines Interactions of Potential Inhibitor with SARS-CoV-2 Protease
Researchers from the Baylor College of Medicine employed previously constructed DNA-encoded chemistry technology (DEC-tec) libraries to identify several candidate molecules that could inhibit the action of Mpro, the main protease of SARS-CoV-2. In a recent study, the researchers described CDD-1713, a new inhibitor to the enzyme Mpro that is involved in propagating the virus. The X-ray crystallographic data, which was collected by Banumathi Sankaran in the Molecular Biophysics and Integrated Bioimaging Division, allowed the researchers to determine that CDD-1713 inhibits the activity of Mpro by binding in the active site of this enzyme.
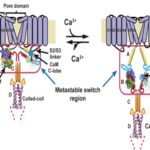
ALS-ENABLE Helps Decode a Calcium-dependent Switch
The Kv7 family of voltage-gated potassium channels control excitability in the heart, brain, and ear, and harbor mutations associated with arrhythmias, epilepsy, and deafness. A recent study, led by Molecular Biophysics and Integrated Bioimaging (MBIB) faculty scientist Daniel Minor’s group in the Cardiovascular Research Institute at UCSF, used both diffraction and scattering beamlines of ALS-ENABLE to reveal a universal switch mechanism by which the calcium sensor protein calmodulin controls the action of these channels. The findings, reported in the journal Neuron, provide a key link between Kv7 channel activity and cellular signaling pathways. Greg Hura, a research scientist in MBIB, was also a co-author on the paper. Watch a video detailing the work.
Was this page useful?