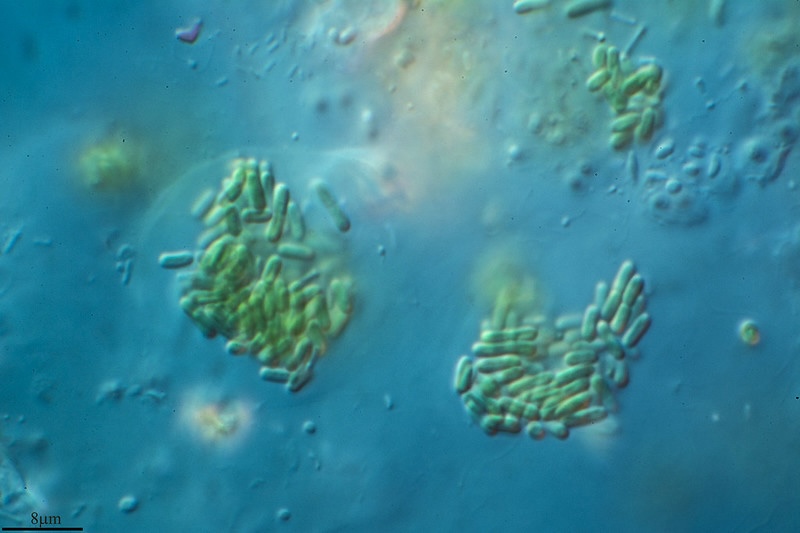
Most of the photosynthesis in oceans is carried out by phytoplankton, tiny single-celled algae that form the base of the marine food web. These microorganisms are susceptible to viral infection, which ultimately causes infected cells to die and become food for other creatures. Until recently, not much was known about what happens when phytoplankton first become infected, however.
Researchers in the Environmental Genomics and Systems Biology (EGSB) Division collaborated on a study led by Sheri Floge, an assistant professor of biology at Wake Forest University, to take a deeper dive and demystify that process. For a paper published in Nature Microbiology, the team employed novel techniques, including a newly developed multiplexed chemotaxis device, with highly controlled viral infection experiments to better understand the ecological role of viruses and virus-infected microbes in ocean systems.
“Our work shows that intact virus-infected cells are biochemically and physiologically different from uninfected cells and that other organisms respond differently to them,” said Floge.
EGSB’s Benjamin Bowen, a staff scientist, Suzanne Kosina, a senior scientific engineering associate, and Trent Northen, a senior scientist and Division Science Deputy, leveraged metabolomics capabilities at the Joint Genome Institute (JGI) to determine the impact of viral infection on dissolved metabolite pools from the marine cyanobacterium Synechococcus. They identified metabolites that could potentially attract bacteria that feed on virus-infected phytoplankton for further testing by collaborators using microfluidic studies.
A better understanding of the role viruses play in these microbial interactions in oceans will aid in the creation of more accurate models of what may happen in the future with climate change.