The projects of eleven Biosciences Area scientists and engineers received funding through the FY2016 Laboratory Directed Research and Development (LDRD) program. These projects cover a broad range of topics, including energy science technology applications, novel computing technologies, and mechanistic understanding of multi-scale interactions among molecules, microbes, plants, metazoans, the abiotic environment, and their feedbacks. Together, these efforts account for nearly 14% of the $25.3 million allocated. Lab-wide, 84 proposals were selected from a field of 179.
Tracking the Onset of Azheimer’s
New research shows for the first time that PET scans can track the progressive stages of Alzheimer’s disease in cognitively normal adults, a key advance in the early diagnosis and staging of the neurodegenerative disorder. William Jagust, senior faculty scientist in the Molecular Biophysics & Integrated Bioimaging Division, is the study’s principal investigator. A video abstract of the findings, published March 2 in the journal Neuron, can be found here. Read more at UC Berkeley News.
Doudna Interview: Biochemist Meets Geneticists
Faculty biochemist Jennifer Doudna recently sat down with Maria Sterrett, a Genetics Society of America (GSA) member, for the Genes to Genomes blog series, “Behind the Podium.” The UC Berkeley professor and HHMI investigator is now famous for her contribution to the development of the CRISPR/Cas9 system for genome editing and will be one of the keynote speakers at The Allied Genetics Conference (TAGC), set to take place in Orlando this July. In the interview, Doudna stressed the pivotal role played in the scientific community by societies like GSA, saying how important it is “to foster a great community of scientists to exchange ideas.” She also observed that societies encourage younger scientists to get involved in the field and introduce them to the cutting edge advances of their field. Read her interview in Genes to Genomes.
Biosciences Researchers Receive Sloan Fellowships
Ke Xu (left) and Wenjun Zhang (right) of the Biosciences Area have been named Alfred P. Sloan Foundation fellows. Xu is a faculty chemist in the Molecular Biophysics & Integrated Bioimaging Division and Zhan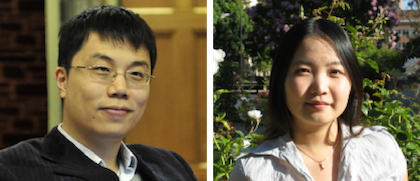 g is a faculty biologist in the Environmental Genomics & Systems Biology Division. These scientists are two of the eight UC Berkeley assistant professors to be honored by the Foundation, along with 118 other new fellows announced today. Fellowships are awarded in eight scientific and technical fields: chemistry, computer science, economics, mathematics, computational and evolutionary molecular biology, neuroscience, ocean sciences and physics. Read more at Berkeley News.
g is a faculty biologist in the Environmental Genomics & Systems Biology Division. These scientists are two of the eight UC Berkeley assistant professors to be honored by the Foundation, along with 118 other new fellows announced today. Fellowships are awarded in eight scientific and technical fields: chemistry, computer science, economics, mathematics, computational and evolutionary molecular biology, neuroscience, ocean sciences and physics. Read more at Berkeley News.
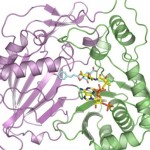
New Way to Reduce Plant Lignin Could Lead to Cheaper Biofuels
Aymerick Eudes and Dominique Loqué of the Joint Bioenergy Institute (JBEI) led a study that shows for the first time that an enzyme can be tweaked to reduce lignin in plants. Their technique could help lower the cost of converting biomass into carbon-neutral fuels to power your car and other sustainably developed bio-products. The crystal structure of this enzyme was solved using data collected in the Berkeley Center for Structural Biology at the Advanced Light Source. Read more on the Berkeley Lab News Center.
- « Previous Page
- 1
- …
- 70
- 71
- 72
- 73
- 74
- …
- 78
- Next Page »
Was this page useful?