Indigo has been prized since antiquity for its vibrancy and deep blue hue and, for more than a century, its unique properties have been leveraged to produce the popular textile blue denim. However, the dyeing process requires chemical steps that are environmentally damaging. A team of researchers in the Molecular Biophysics and Integrated Bioimaging (MBIB) and Biological Systems and Engineering (BSE) Divisions, at JBEI, and UC Berkeley have developed a promising sustainable indigo dyeing process that relies on genetically engineered bacteria, mimicking the natural biochemical protecting group strategy employed by the Japanese indigo plant Polygonum tinctorium.
Crystallization Screen Created by Berkeley Lab Biosciences Scientists Reaches the Market
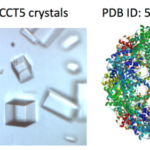
 X-ray crystallography has been the most successful technique used to solve macromolecular structures, contributing several thousand new entries to the Protein Data Bank (PDB) every year. The protein crystal is the critical starting point for X-ray data collection, and consequently, its properties are correlated with the quality of the data and the level of detail that can be extracted for a macromolecular structure. However, proteins require solutions of specific composition to form crystals for structure determination studies. These specifications are usually determined from exposing the protein to several different solutions in a crystallization screen.
X-ray crystallography has been the most successful technique used to solve macromolecular structures, contributing several thousand new entries to the Protein Data Bank (PDB) every year. The protein crystal is the critical starting point for X-ray data collection, and consequently, its properties are correlated with the quality of the data and the level of detail that can be extracted for a macromolecular structure. However, proteins require solutions of specific composition to form crystals for structure determination studies. These specifications are usually determined from exposing the protein to several different solutions in a crystallization screen.
A team of researchers in the Molecular Biophysics and Integrated Bioimaging (MBIB) Division led by Paul Adams and Jose Henrique Pereira have developed a new crystal screen, the Berkeley Screen, with 96 conditions proven to be highly effective at producing crystals for structural determination. The Berkeley Screen is now available to the wider crystallography community commercially.
Tracking Energy Flow in Light-harvesting Systems on Native Nanometer and Picosecond Scales
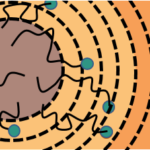
In the first trillionths of a second after sunlight hits a photosynthetic organism, the energy that is absorbed flows through a dense network of protein-bound chlorophyll molecules to a dedicated location where it is converted to electric charges. This is the first step in a series of events that ultimately drives the formation of sugar and starch to store energy in chemical bonds.
“This migration is the triggering event that leads to all of the oxygen that we breathe, all of the food that we have, and we really don’t understand why this part of photosynthesis works as well as it does. For every photon of light that’s absorbed, you can expect some biochemical action to occur. That efficiency is really remarkable,” says Naomi Ginsberg, a faculty scientist in the Molecular Biophysics and Integrated Bioimaging (MBIB) Division who has a secondary affiliation in Materials Sciences and is also a UC Berkeley associate professor of Chemistry and Physics.
Ginsberg and her colleagues devised a way to measure migration efficiency, and they describe the method in Nature Materials in November 2017.
Super-Resolution Microscopy Reveals Fine Detail of Cellular Mesh
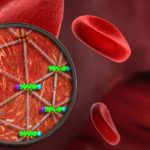
Ke Xu, faculty scientist in the Molecular Biophysics and Integrated Bioimaging Division, used super-resolution microscopy to reveal the geodesic mesh supporting red blood cells, enabling them to be sturdy yet flexible enough to squeeze through narrow capillaries as they carry oxygen to tissues. The discovery could help uncover how malaria parasites hijack this mesh and destroy red blood cells. Read more at UC Berkeley News.
Silencing Is Golden: Scientists Image Molecules Vital for Gene Regulation
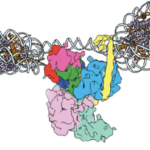
In two new studies, a team of researchers led by Eva Nogales, senior faculty scientist in the Molecular Biophysics and Integrated Bioimaging (MBIB) Division, has gained insight into the structure and function of molecules that act at the genetic level to give rise to different types of cells.
- « Previous Page
- 1
- …
- 46
- 47
- 48
- 49
- 50
- …
- 77
- Next Page »
Was this page useful?