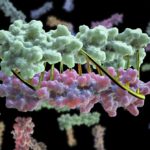
Bioscientists at the Advanced Light Source (ALS) at Berkeley Lab lent their expertise to a project led by scientists at the University of Washington to design proteins in the lab that zip together like DNA. The technique could enable the design of protein nanomachines to help diagnose and treat disease, allow for more precise engineering of cells, and perform a variety of other tasks.
Berkeley Lab Bioscientists Participate in CASP13
CASP is the Critical Assessment of Protein Structure Predictions, a biannual “competition” to determine which prediction algorithm generates the most accurate model. There are several categories in which models will be assessed, including accuracy, topology, and biological relevance. The SIBYLS beamline is participating to provide small-angle X-ray scattering (SAXS) data for––and judging for the first time––the “data-assisted” category. This CASP competition should lead to improvement in predicting protein-protein interfaces and complex structures.
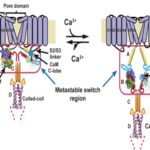
ALS-ENABLE Helps Decode a Calcium-dependent Switch
The Kv7 family of voltage-gated potassium channels control excitability in the heart, brain, and ear, and harbor mutations associated with arrhythmias, epilepsy, and deafness. A recent study, led by Molecular Biophysics and Integrated Bioimaging (MBIB) faculty scientist Daniel Minor’s group in the Cardiovascular Research Institute at UCSF, used both diffraction and scattering beamlines of ALS-ENABLE to reveal a universal switch mechanism by which the calcium sensor protein calmodulin controls the action of these channels. The findings, reported in the journal Neuron, provide a key link between Kv7 channel activity and cellular signaling pathways. Greg Hura, a research scientist in MBIB, was also a co-author on the paper. Watch a video detailing the work.
NIH Awards $6.5 Million for Augmenting Structural Biology Research Experience
The National Institutes of Health (NIH) has awarded $6.5 million to Berkeley Lab to integrate existing synchrotron structural biology resources to better serve researchers. The grant will establish a center based at the Lab’s Advanced Light Source (ALS) called ALS-ENABLE that will guide users through the most appropriate routes for answering their specific biological questions.
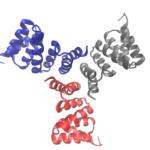
Designing Cyclic Oligomers: Greater than the Sum of Their Parts
Cyclic proteins that assemble from multiple identical subunits (homo-oligomers) play key roles in many biological processes, including enzymatic catalysis and function and cell signaling. Researchers in the Molecular Biophysics and Integrated Bioimaging (MBIB) Division worked with University of Washington’s David Baker, who led a team to design in silico and crystallize self-assembling cyclic homo-oligomer proteins.
Was this page useful?