Claire Tomlin, a biological faculty engineer in the Biological Systems and Engineering (BSE) Division, has been elected to the American Academy of Arts and Sciences. The prestigious 239-year old honorary society recognizes accomplished scholars, scientists, and artists in academia, the humanities, arts, business, and government. Tomlin’s research, which is currently conducted primarily at UC Berkeley, where she is a professor of electrical engineering and computer sciences, explores complex systems that have discrete event dynamics as well as continuous time dynamics. Her group studies many topics and problems that can be modeled by hybrid systems as well as more general robotics, such as air traffic control automation, algorithms for decentralized optimization, modeling and analysis of biological cell networks, and unmanned aerial vehicle design and control. The 2019 class of 200-plus new lifetime members announced this week will be inducted at a ceremony in October 2019 in Cambridge, Massachusetts.
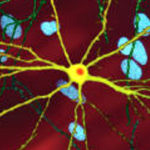
Astrocyte Insight Explains Brain Region-specific Vulnerability in Huntington Disease
The mutant form of the Huntington gene, mHTT, which encodes a product that causes the disease, is expressed throughout the brain in affected individuals. Yet neurons in individual regions of the brain are differentially susceptible to its neurotoxic effects. The basis for this puzzling region-specific vulnerability in Huntington disease—which is likewise a feature of Alzheimer and Parkinson neurodegenerative diseases—was hitherto unknown.
A new study led by Cynthia McMurray, a senior scientist in Molecular Biophysics and Integrated Bioimaging (MBIB), provides evidence that regional differences in neuronal susceptibility to Huntington disease can be attributed to substrate-driven metabolic reprogramming strategies adopted by astrocytes in response to low glucose. The team recently reported their findings in the journal Cell Metabolism.
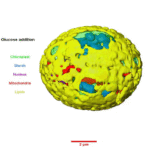
Regulation of Algal Photosynthesis and Metabolism
The unicellular green alga Chromochloris zofingiensis has the ability to shift metabolic modes from photoautotrophic (synthesizing food using light as energy source) to heterotrophic (obtaining food and energy from exogenous sources) in response to carbon source availability in the light. It also has the capacity—under certain conditions—to produce high amounts of commercially relevant bioproducts: notably, the ketocarotenoid astaxanthin, used in feed, cosmetics, and as a nutraceutical, and triacylglycerol (TAG) biofuel precursors.
Understanding how photosynthesis and metabolism are regulated in algae could, via bioengineering, enable scientists to reroute metabolism toward beneficial bioproducts for energy, food, and human health. To that end, Berkeley Lab Biosciences researchers used C. zofingiensis as a simple algal model system to investigate conserved eukaryotic sugar responses, as well as mechanisms of thylakoid breakdown and biogenesis in chloroplasts.
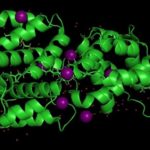
First Look at New Light Absorbing Protein
The Helical Carotenoid Protein 2 (HCP2) protein is an ancestor of proteins that are known to protect against damage caused by excess light exposure. Researchers in the laboratory of Cheryl Kerfeld, guest faculty in the Environmental Genomics & Systems Biology (EGSB) Division, are the first to structurally and biophysically analyze a protein from the HCP family. This HCP protein family was discovered recently by Kerfeld and the members of her lab, who are based in EGSB and at Michigan State University (MSU). To solve the molecular structure of HCP2, X-ray diffraction was measured at beam line 5.0.2 in the Berkeley Center for Structural Biology of the Advanced Light Source (ALS). The structure was refined using Phenix, a software suite for automated determination of molecular structures developed under the direction of Paul Adams, Molecular Biophysics and Integrated Bioimaging Division Director. Read more in the MSU-DOE Plant Research Laboratory news story.
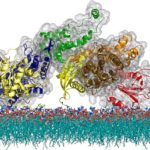
How T Cells Tune Out Fake Signals: Phase Transition Timing Is Everything
A group of Berkeley Lab and UC Berkeley physical chemists led by Jay Groves, faculty scientist in Molecular Biophysics and Integrated Bioimaging (MBIB), has—for the first time—imaged the process by which an individual immune system molecule is switched on in response to a signal from the environment. This breakthrough led to the discovery that the immune system activation process involves hundreds of proteins suddenly coming together to form a linked network through a process known as phase transition. Critically, the process has a built in time delay which allows the cell to distinguish a genuine receptor stimulation from background chemical noise. The work is described in a paper recently published in the journal Science.
- « Previous Page
- 1
- …
- 97
- 98
- 99
- 100
- 101
- …
- 213
- Next Page »
Was this page useful?