Coronaviruses—a group that includes severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS)—are covered with spikey protein projections that give the appearance of a crown. These spikes help the virus fuse with a host cell so it can take control of cell’s machinery to replicate itself. Currently, no specific treatments or vaccines are available against any of the six human-infecting coronaviruses.
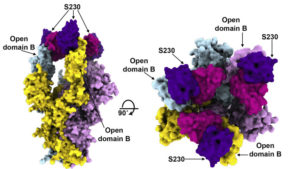
Orthogonal views of the SARS spike protein in complex with three neutralizing antibody fragments from combined cryo-EM and crystallography data.
To better understand how coronavirus antibodies work, a team of researchers at the University of Washington studied spike protein structures in complex with neutralizing antibody fragments isolated from SARS and MERS survivors. To visualize how the spike structures interact with the antibody fragments, they used a cryo-electron microscopy (cryo-EM) for the spikes, which are resistant to crystallization, and protein crystallography for the fragments. The high-resolution x-ray crystallography was performed at the Advanced Light Source (ALS) Beamline 5.0.1, part of the Berkeley Center for Structural Biology (BCSB).
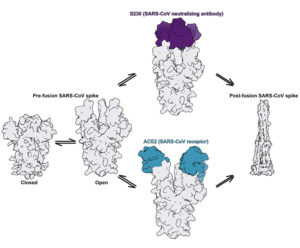
A SARS antibody (purple) mimics the function of the virus’s natural receptor (teal), prematurely triggering (and thus deactivating) the mechanism that enables the SARS virus to infect a cell.
Upon fitting the fragment crystal structures into the cryo-EM maps of the spikes, the researchers discovered an unprecedented example of functional mimcry. The SARS antibody was found to induce structural changes in the spike similar to those produced by its natural receptor (ACE2), changes known to be necessary for initiating membrane fusion. The results provide valuable insights into how antibodies confer broad protection against coronavirus strains that can inform efforts to prevent and treat these serious, often deadly, respiratory diseases.
Read more in the ALS Science Highlight.




