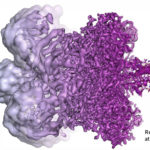
Several Berkeley Lab researchers contributed to the foundational work leading to the development of cryo-electron microscopy (cryo-EM), for which Jacques Dubochet, Joachim Frank, and Richard Henderson were awarded the 2017 Nobel Prize in Chemistry. Among those cited in the Scientific Background are Biosciences’ Bob Glaeser, Ken Downing, and Eva Nogales.
New Addition to Family of Light-responsive Proteins
A study led by Cheryl Kerfeld, with colleagues from Biosciences’ Molecular Biophysics and Integrated Bioimaging (MBIB) Division, as well as her MSU-DOE Plant Research group at Michigan State University, made the cover of the August 2017 issue of Nature Plants. Matthew Melnicki, Markus Sutter, and Fei Cai of MBIB contributed to the study, which characterized a recently identified member of the orange carotenoid family of proteins (OCPs). These proteins change conformation in response to ambient light conditions to protect the host cyanobacteria from harmful exposure. Compared to the canonical exemplar, OCP1, the new OCP, called OCP2, requires relatively higher light intensity for activation, but it reacts faster than OCP1. The goal of Kerfeld’s OCP research is to understand how the various members of the family work, and use that knowledge to engineer the protein for applications in renewable energy and medicine. Read more from the MSU-DOE Plant Research Lab.
Carolyn Larabell to Receive Shirley Award at ALS User Meeting
Carolyn Larabell, a professor at the UC San Francisco School of Medicine and a faculty scientist in the Molecular Biophysics and Integrated Bioimaging (MBIB) Division at Berkeley Lab, has been selected by the Advanced Light Source (ALS) Users’ Executive Committee to receive the 2017 David A. Shirley Award for Outstanding Scientific Achievement. Larabell directs the National Center for X-Ray Tomography (NCXT) centered around ALS Beamline 2.1, the world’s first soft x-ray microscope for biological imaging, and she led the group responsible for its commissioning, design, and construction. Soft x-ray tomography makes it possible to image intact biological cells in 3-D without structure-damaging preparation such as chemically fixing, dehydrating, and staining the specimens. On October 3, Larabell will give her award talk, entitled “The Expanding Universe of Cell Biology at the NCXT,” during the ALS User Meeting at Berkeley Lab. Read more in ALS News & Updates.
UCB/UIUC Project to Improve Photosynthesis Receives Ongoing Support
A University of Illinois Urbana-Champaign research initiative on which Biosciences’ Krishna Niyogi has been a longtime collaborator received a $45 million, five-year reinvestment to continue efforts to re-engineer photosynthesis in staple crops order to sustainably increase yields worldwide. The new round of funding for Realizing Increased Photosynthetic Efficiency (RIPE) comes from the Bill & Melinda Gates Foundation, the Foundation for Food and Agriculture Research, and the U.K. Department for International Development. Niyogi, a faculty scientist in Molecular Biophysics and Integrative Bioimaging (MBIB) and chair of the Department of Plant and Microbial Biology at UC Berkeley, has been involved with RIPE since its inception. His research related to the project focuses on relaxing the protective mechanisms plants have developed to avoid damage to leaves from high-intensity light. Read more from UC Berkeley College of Natural Resources.
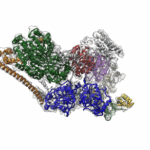
A Near-Atomic Resolution Map of a Key DNA Protein Complex
Molecular Biophysics and Integrated Bioimaging (MBIB) Division scientists led by Eva Nogales have resolved the 3-D structure of a critical human cellular protein complex involved in DNA transcription and repair at an unprecedented level of resolution. The complex, called transcription factor IIH (TFIIH), unzips the DNA double helix so that genes can be accessed and read. Malfunctions of the complex are associated with premature aging, cancer propensity, and a variety of other defects. One challenge with solving the structure of TFIIH has been that it exists in such minute amounts that it is difficult to produce and purify in large quantities. Moreover, once obtained, it may not form crystals suitable for X-ray diffraction. The researchers used cryo-electron microscopy (cryo-EM), a technique in which purified samples are flash-frozen at ultra cold temperatures, and which works even on very small quantities. “The fact that we resolved this protein structure from human cells makes this even more relevant to disease research,” said Nogales. Basil Greber, a postdoctoral fellow in Nogales’s lab, was first author on the study published in the journal Nature. Computational research scientist Pavel Afonine and MBIB Division Director Paul Adams also contributed to the project. Read more from the Berkeley Lab News Center.
- « Previous Page
- 1
- …
- 50
- 51
- 52
- 53
- 54
- …
- 78
- Next Page »
Was this page useful?