Conveyor Belt Innovation Speeds Up Biological Discovery
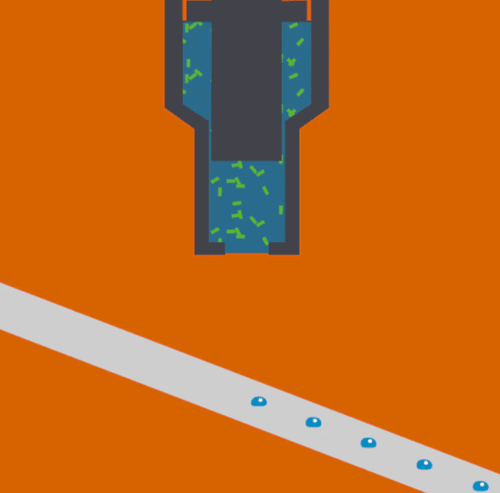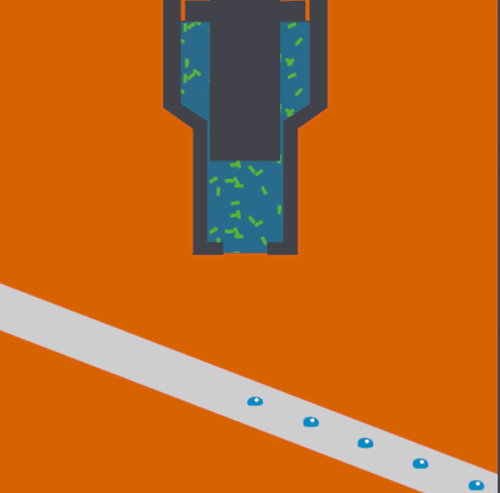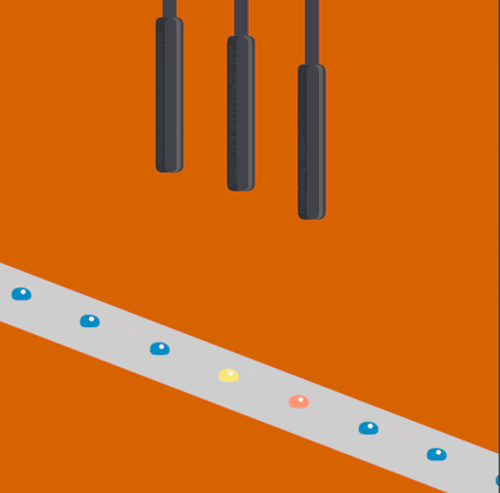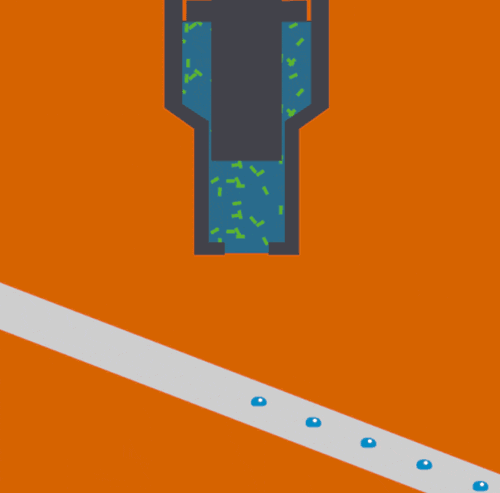
Bioscientists Junko Yano, Vittal Yachandra, and Jan Kern led a multi-institutional collaboration to develop and demonstrate a novel Acoustic Droplet Ejection-“Droplet on Tape” method of sample delivery for free electron laser experiments. The study, which was published yesterday in Nature Methods, describes the techniques that were deployed at SLAC’s Linac Coherent Light Source.
More »

 Seven LBNL Biosciences Area researchers are among the 47 new investigators chosen by the
Seven LBNL Biosciences Area researchers are among the 47 new investigators chosen by the 



