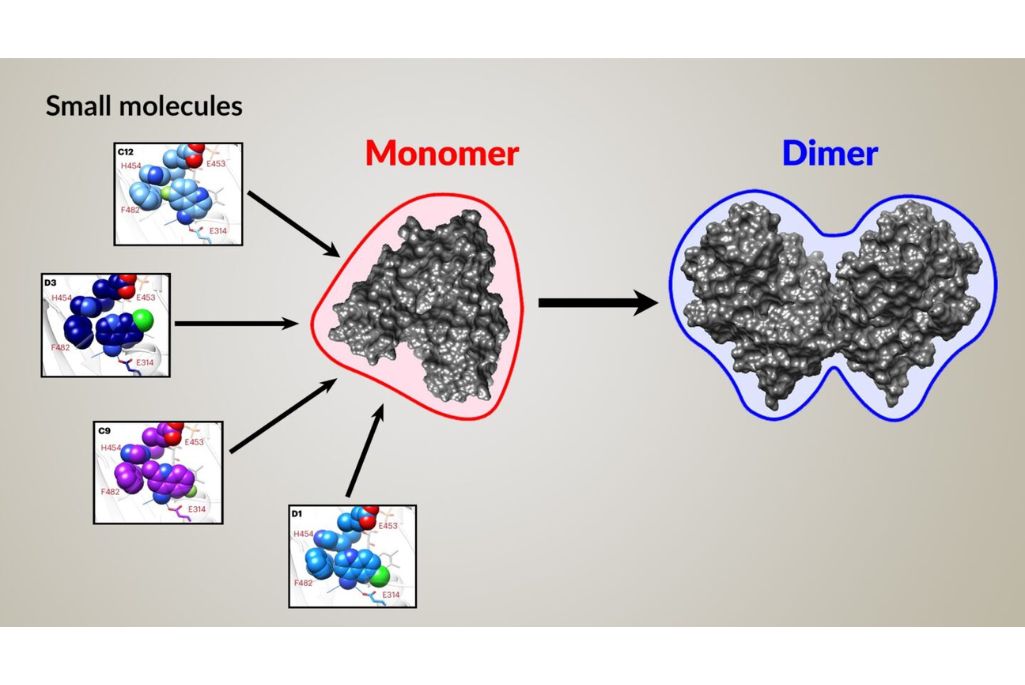
When screening small molecules as potential drug candidates, it’s important to understand how they affect a target protein’s biological function, which is usually connected to its physical arrangement. A team of researchers led by Chris Brosey of the University of Texas MD Anderson Cancer Center developed a high-throughput drug-discovery workflow leveraging time-resolved small-angle X-ray scattering (SAXS) capabilities at the Advanced Light Source’s (ALS) Structurally Integrated Biology for the Life Sciences (SIBYLS) beamline 12.3.1.
In this case, the researchers were looking for small molecules that would stimulate the conformational change of a protein involved in programmed cell death (apoptosis) from a monomer into a dimer, thereby inhibiting the protein. They used the pipeline to screen 2,500 small-molecule drug candidates for this ability.
John Tainer, a professor at the University of Texas MD Anderson Cancer Center and guest faculty in the Molecular Biophysics and Integrated Bioimaging (MBIB) Division, assisted with the study’s conception and data analysis and was a co-corresponding author on the paper published in Nature Chemical Biology. Greg Hura, MBIB Division Deputy for Science, member of the SIBYLS group, and co-principal investigator of the ALS Efficiently Networking Advanced BeamLine Experiments (ALS-ENABLE) program, and Kathryn Burnett, an administrator with ALS-ENABLE, collected and processed the high-throughput, time-resolved SAXS data.