“Viral factories” are areas in virus-infected host cells where the tools and materials necessary for viral replication are concentrated. A team led by researchers at the Baylor College of Medicine sought to learn more about a protein called σNS, an important component of some viral factories. This protein takes part in the replication of reoviruses, which do not generally cause disease and can be used as to infect and break down cancer cells. Despite the protein’s importance, the underlying mechanics of σNS have remained unclear.
The multi-institutional team, which included Banumathi Sankaran, a research scientist in the Molecular Biophysics and Integrated Bioimaging Division, published their results in Nature Communications earlier this year. Sankaran leads the Collaborative Crystallography (CC) mail-in service at the Advanced Light Source (ALS), which is run under the aegis of the ALS-ENABLE program. She collected X-ray diffraction data for crystals analyzed using beamlines in the Berkeley Center for Structural Biology at the ALS.
Because σNS resists crystallization, the researchers looked at a mutant version, σNS-R6A, which forms dimers rather than the longer chains (oligomers) of the un-mutated protein.
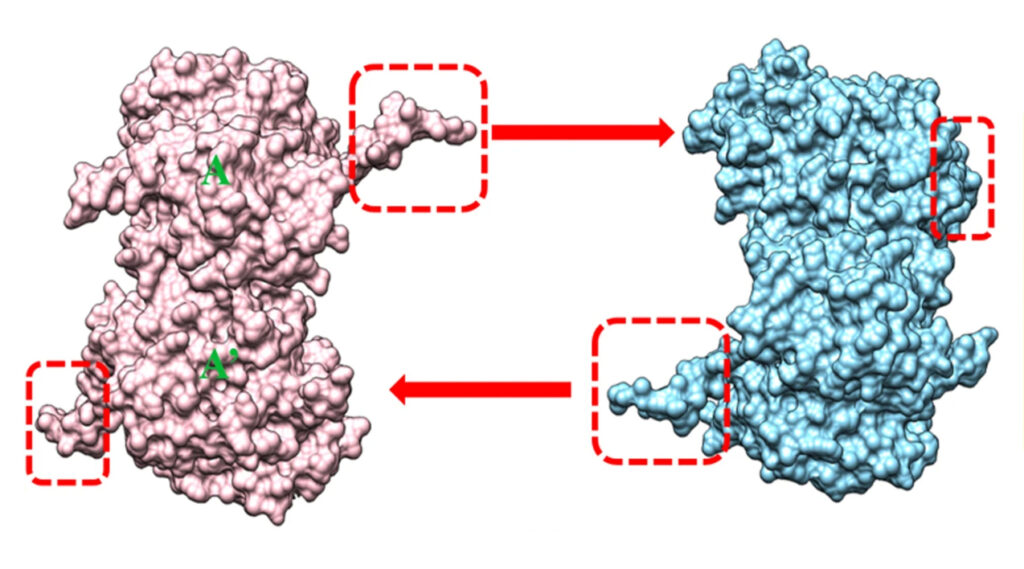
Observations from studies with bile acids, together with structural and biochemical studies of mutated versions of σNS, provided the scientists insights into the function of the σNS protein in reovirus replication. Understanding these mechanisms will foster development of therapeutic strategies against viruses that use similar proteins to make more copies of themselves and continue the infection cycle.
This article was adapted from an ALS Science Brief.



