The field of biotechnology enlists microbes such as yeast and E. coli to produce medicines and other bioproducts. When it comes time to purify these valuable products, though, the fatty compounds that envelope all living cells are one of the most problematic contaminants. “Fat-free” synthetic cells could be used to produce myriad useful products and provide a deeper understanding of fundamental biological principles.
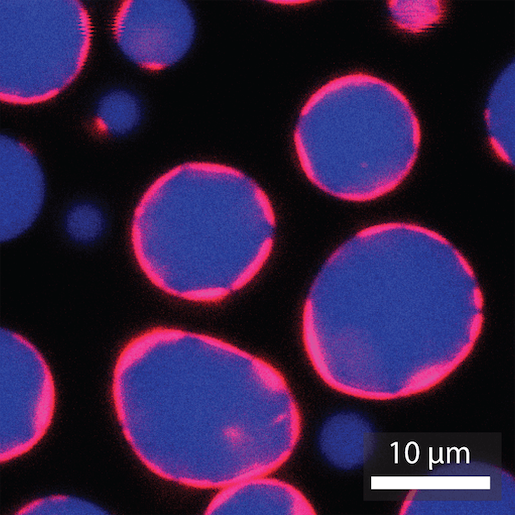
Cheryl Kerfeld’s laboratory, which operates at both Michigan State University and Berkeley Lab, teamed up with researchers at Pennsylvania State University and the University of Delaware to take a first step toward creating artificial cells that lack a lipid membrane. Drawing inspiration from bacteria and forces at work in the biological world, the team created protein-coated shells that can be packed with active cargo including enzymes and RNA.
At MSU, Kerfeld is a distinguished professor in the Department of Biochemistry and Molecular Biology and a member of the MSU-Department of Energy Plant Research Laboratory. At Berkeley Lab, she is a guest faculty member in Biosciences’ Environmental Genomics and Systems Biology (EGSB) Division. She has extensive experience working with bacterial microcompartments (BMCs), which operate as organelles inside bacteria, where their protein shells isolate their inner workings from their surroundings.
For this project, Kerfeld and colleagues used the proteins from BMCs to create much larger compartments—structures the size of cells—and demonstrated that they could carry four different types of cargo inside. The team is eager to do further experiments to characterize the shells and learn how to control various parameters, such as their size, shape, and thickness. Eventually, they plan to tinker with the protein composition of the enclosures to give them different functionalities, and prototype different cargo that caters to medicine, bioenergy, and space applications.



