Researchers develop an efficient method for studying fast biochemical reactions as they happen in real time.
As part of an international collaboration, researchers at Lawrence Berkeley National Laboratory (Berkeley Lab), the Diamond Light Source synchrotron facility, and Oxford and Bristol Universities in England have developed a novel sample delivery system that expands the limited toolkit for performing dynamic structural biology studies of enzyme catalysis, which have so far mostly been limited to a small number of light-driven enzymes.
Enzymes are molecules – primarily proteins – that tremendously accelerate biochemical reactions by selectively binding to target molecules, or substrates. Without enzymes, most biological reactions simply could not occur under temperature and pressure conditions compatible with life.
Earlier experiments have been successful in describing the time-course of these reactions, but knowledge about how the structure of the catalyst changes during the reactions has been lacking, explained Jan Kern, a staff scientist in the Biosciences Area at Berkeley Lab and one of the study’s lead authors.

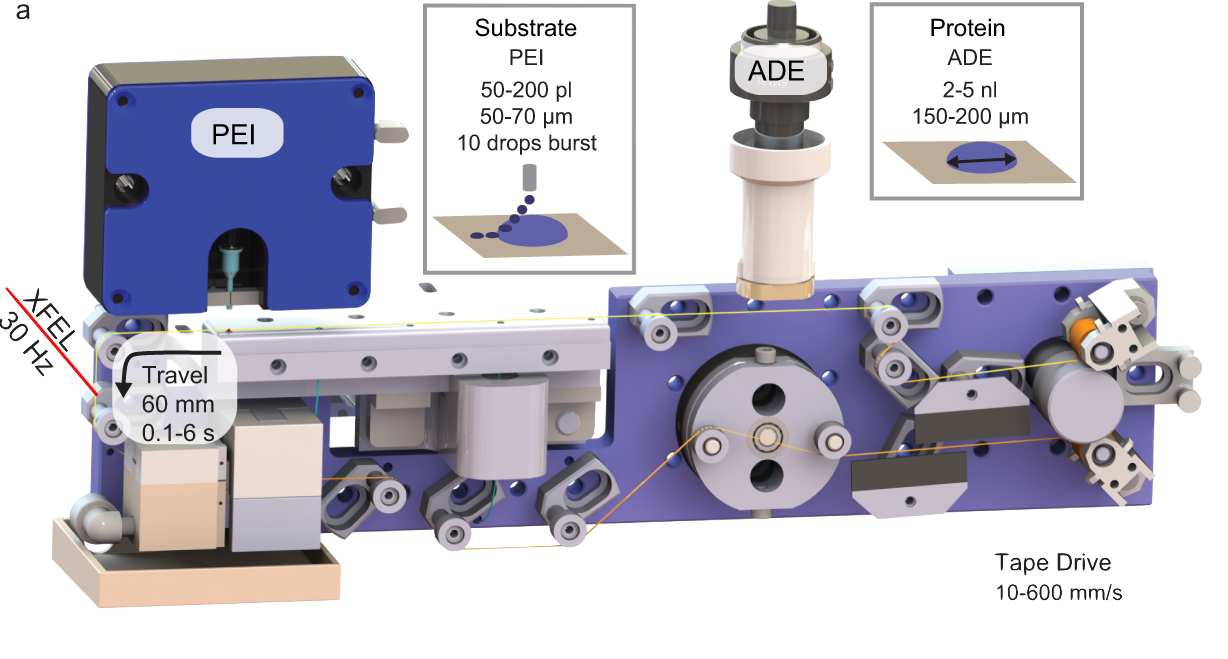
A moving tape carries microscopic dabs of crystalized enzyme slurry, to which even tinier droplets of substrate are added via a second nozzle. The turbulence caused by the barrage of smaller droplets colliding with the larger one leads to rapid, even mixing so the enzyme-substrate reactions begin simultaneously throughout the sample.
The new technique builds on the team’s innovative approach of depositing microscopic dabs of crystalized enzyme slurry on a moving tape that, like a conveyor belt, carries the samples to the beam of an X-ray free-electron laser (XFEL). The researchers modified the so-called “drop on tape” design to accommodate a second nozzle to deposit a variable number of picoliter-volume (one-trillionth of a liter) droplets of substrate on top of a larger, nanoliter-volume (one-billionth of a liter) drop of enzyme microcrystal slurry.
The turbulence caused by the barrage of smaller droplets colliding with the larger one leads to rapid, even mixing so the enzyme-substrate reactions begin simultaneously throughout the sample. This makes it possible to collect serial diffraction data over a range of time, from ~100 ms to several seconds after the enzyme and substrate begin to interact to create a time-resolved “molecular movie” of the reaction cycle.
The team validated their “drop-on-drop” technique at the SACLA XFEL source in Japan using two different enzyme systems. “Our new approach allows us to follow steps of reactions in enzymes that we were not able to visualize previously. For example, we were able to see how an antibiotic drug molecule is binding to a beta lactamase, an enzyme present in antibiotic resistant bacteria that can inactivate the antibiotic,” said Kern.
Additional MBIB researchers who contributed to this work include: project scientist Asmit Bhowmick, graduate student research assistant Isabel Bogacz, research scientist Aaron Brewster, senior scientist Frances Houle. postdoctoral scholar In-Sik Kim, undergraduate research assistant Ramzi Massad, senior scientist Nicholas Sauter, postdoctoral scholar Philipp Simon, senior scientist Vittal Yachandra, and Interim Division Director Junko Yano.
This Science Snapshot was published on the Berkeley Lab News Center.