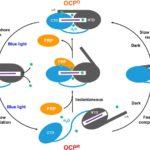
Researchers at Berkeley Lab and Michigan State University (MSU), led by Corie Ralston and Cheryl Kerfeld, performed X-ray footprinting mass spectrometry (XFMS) experiments at the Advanced Light Source (ALS) beamline 5.3.1, which revealed new mechanistic details of the key events in orange carotenoid protein (OCP) photoprotection. XFMS is ideally suited to probing conformational dynamics at the single residue level, providing both a spatial and temporal view of site-specific changes in the OCP and its interaction with the fluorescence recovery protein (FRP). The experiments showed that FRP provides an extended binding region that holds the OCP together and forces proximity of the two domains that accelerate relaxation of OCP to its native state.
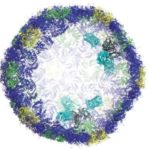
A Synthetic Nanofactory Inspired by Nature
Researchers at Berkeley Lab and Michigan State University (MSU), led by Cheryl Kerfeld, have created a genetically engineered bacterial microcompartment (BMC) shell based on natural structures and the principles of protein evolution. The new shell is smaller and simpler, made of only a single designed protein (natural BMCs are made of up to three), making it easier to work with in the lab.
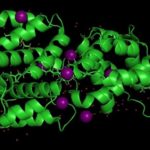
First Look at New Light Absorbing Protein
The Helical Carotenoid Protein 2 (HCP2) protein is an ancestor of proteins that are known to protect against damage caused by excess light exposure. Researchers in the laboratory of Cheryl Kerfeld, guest faculty in the Environmental Genomics & Systems Biology (EGSB) Division, are the first to structurally and biophysically analyze a protein from the HCP family. This HCP protein family was discovered recently by Kerfeld and the members of her lab, who are based in EGSB and at Michigan State University (MSU). To solve the molecular structure of HCP2, X-ray diffraction was measured at beam line 5.0.2 in the Berkeley Center for Structural Biology of the Advanced Light Source (ALS). The structure was refined using Phenix, a software suite for automated determination of molecular structures developed under the direction of Paul Adams, Molecular Biophysics and Integrated Bioimaging Division Director. Read more in the MSU-DOE Plant Research Laboratory news story.
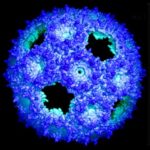
New Ways to Control Bacterial Factories for Future Biotech Uses
A team of researchers led by Cheryl Kerfeld, faculty affiliate in the Environmental Genomics & Systems Biology (EGSB) Division, have developed a new method to manipulate miniature factories found in bacteria that could someday lead to new medical, industrial, or energy applications. The factories, called bacterial microcompartments – or BMCs – are found in bacteria all over the world.
New Methods to Control Bacterial Factories for Biotech Aims
Bacterial microcompartments (BMCs) are organelles that encapsulate portions of metabolic pathways, like miniature factories. They’re found across diverse phyla and do different things depending on the host. Scientists want to retrofit these factories to perform desired functions, such as producing biofuels, industrial materials, or nanoscale medical devices. But current technologies to manipulate BMCs, which consist of an enzymatic core surrounded by a shell made up of protein tiles, have limitations. In a recently published Nature Communications paper, researchers affiliated with Berkeley Lab’s Environmental Genomics and Systems Biology (EGSB) Division and Michigan State’s MSU-DOE Plant Research Laboratory present two new methods they’ve developed to facilitate the construction of synthetic versions.
Was this page useful?