Bacteria deploy various biological mechanisms to fend off their competition, which are often other bacteria. In some cases, they secrete toxins in their fight for survival. A newly discovered toxin stands out from others in the battle for microbial domination. Marc Allaire, Molecular Biophysics & Integrated Bioimaging (MBIB) Division researcher, worked with a team led by Joseph Mougous of the Howard Hughes Medical Institute and University of Washington School of Medicine, to characterize this new toxin.
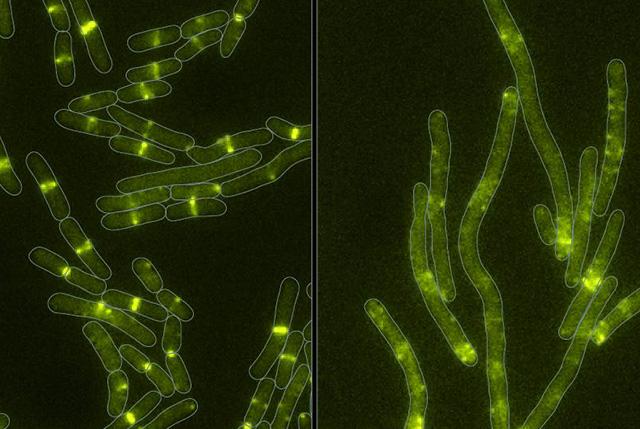
Healthy bacteria (left) and bacteria (right) whose cell-division machinery has been disrupted by a toxin newly discovered in some bacterial arsenals. (Image credit: Mougous Lab)
While many deadly substances have been identified among bacteria, this previously unknown toxin behaves in a familiar way. In a research article published in the November 15 issue of Cell, the scientists describe the mechanism of action and their finding that it works like the villainous toxins of human pathogens such as pertussis, cholera, and diphtheria.
Allaire, a beamline scientist and crystallographer from the Berkeley Center for Structural Biology (BSCB) at the Advanced Light Source (ALS), contributed to this study by collecting the data used to solve the three-dimensional molecular structure of this toxin, Tre1. Beamline 5.0.2 at the ALS, a particle accelerator that generates the bright beams of X-ray light, was used to collect the critical diffraction data from the protein crystals. The ALS is a DOE Office of Science User Facility.
Further studies showed that this toxin targets a protein that is essential for cells to divide. In addition, researchers discovered how the bacterium protects itself from its own weapon and similar attacks from other bacteria.
“It was a privilege to work with Dr. Mougous on this full-circle study,” said Allaire. “One of the benefits of working at the BCSB is the opportunity to participate in great science like this.” To learn more about this new toxin and its mechanism of action, read the University of Washington press release.