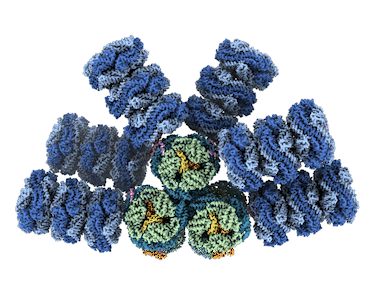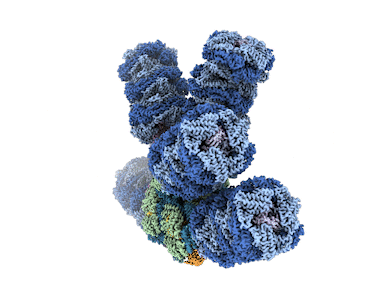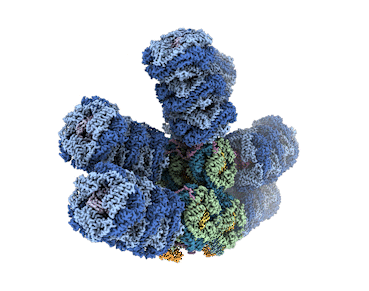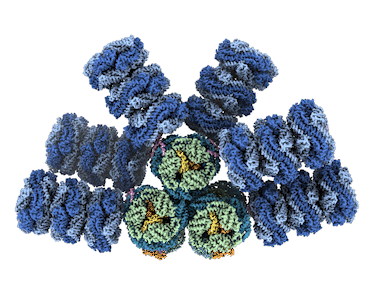
Microbes called cyanobacteria, also known as blue-green algae, are the evolutionary descendants of the first organisms on Earth capable of converting sunlight, water, and carbon dioxide (CO2) into sugars and oxygen. For decades, researchers have sought to understand how cyanobacteria capture light energy via structures called phycobilisomes, and send to where it’s needed to power the conversion. Historically, researchers have been unable to get high-resolution images of these large, fragile complexes of pigments and proteins while they’re intact.
Now, however, advances in cryogenic electron microscopy (cryo-EM) have enabled an international team of experts to visualize the structure of a cyanobacterial phycobilisome with nearly atomic resolution. The work, a collaboration among researchers at Michigan State University (MSU), UC Berkeley, Berkeley Lab, and the University of South Bohemia in the Czech Republic, was published in Nature. Knowing the position of different proteins and pigments helps scientist better understand this natural process and can inspire future applications in areas such as renewable energy and environmental remediation.
“There’s a lot of interest in using cyanobacteria as solar-powered factories that capture sunlight and convert it into a kind of energy that can be used to make important products,” said Cheryl Kerfeld, a member of the MSU-DOE Plant Research Laboratory, which is supported by the U.S. Department of Energy. “With a blueprint like the one we’ve provided in this study, you can start thinking about tuning and optimizing the light-harvesting component of photosynthesis.”
“Once you see how something works, you have a better idea of how you can modify it and manipulate it. That’s a big advantage,” said Markus Sutter, a senior research associate in the Kerfeld Lab, which operates at MSU and Berkeley Lab, and affiliate scientist in Environmental Genomics and Systems Biology (EGSB).

The team documented several new findings, including finding a new phycobilisome protein and observing two new ways that the phycobilisome orients its light-capturing rods that hadn’t been resolved before.
“It’s 12 pages of discoveries,” said María Agustina Domínguez-Martín of the Nature report. As a postdoctoral researcher in the Kerfeld Lab, Domínguez-Martín initiated the study at MSU and brought it to completion at Berkeley Lab.
“This work is a breakthrough in the field of photosynthesis,” said Paul Sauer, a postdoctoral researcher in UC Berkeley Professor Eva Nogales’ cryo-EM lab. Nogales is also a senior faculty scientist in the Molecular Biophysics and Integrated Bioimaging (MBIB) Division. “Our discovery helps us understand how evolution came up with ways to turn CO2 and light into oxygen and sugar in bacteria, long before any plants existed on our planet.”
The insights could also help researchers remediate harmful algal blooms, develop artificial photosynthesis systems for renewable energy, and enlist microbes in sustainable manufacturing that starts with the raw materials of CO2 and sunlight. Beyond the new answers and potential applications this work offers, the researchers are also excited about the new questions this work raises and the research it could inspire.
Read more in the Berkeley Lab News Center.