A team led by Eva Nogales, senior faculty scientist in the Molecular Biophysics and Integrated Bioimaging (MBIB) Division, has produced the first detailed 3D structure of human SAGA, a 20-piece molecular machine that’s crucial to life.
Errors in this protein complex contribute to distinctly human problems, including autism, neurological disorders, and cancers such as melanoma. Yet research on SAGA has long focused on the simpler yeast version; scientists reported yeast SAGA’s structure in 2020. “We often look at the molecular machinery of organisms like yeast to understand how it functions in humans and how errors can cause disease,” said Nogales, who is also a Howard Hughes Medical Institute (HHMI) investigator and professor at UC Berkeley. But she and her team suspected they would find key differences between the yeast and human structures.
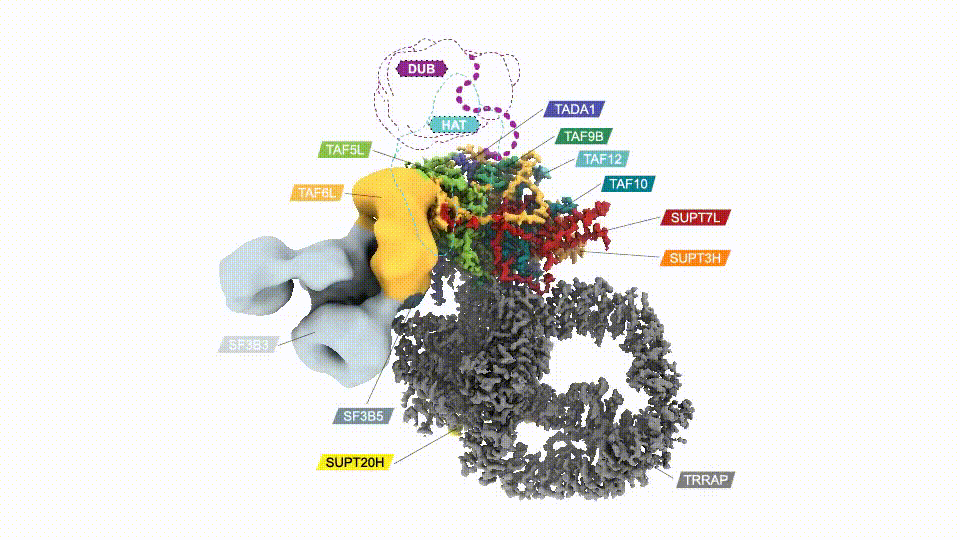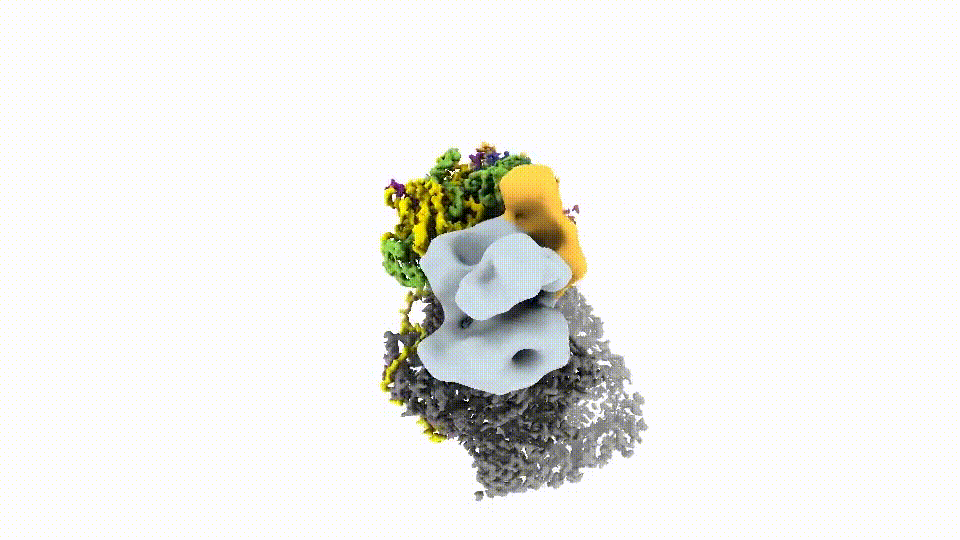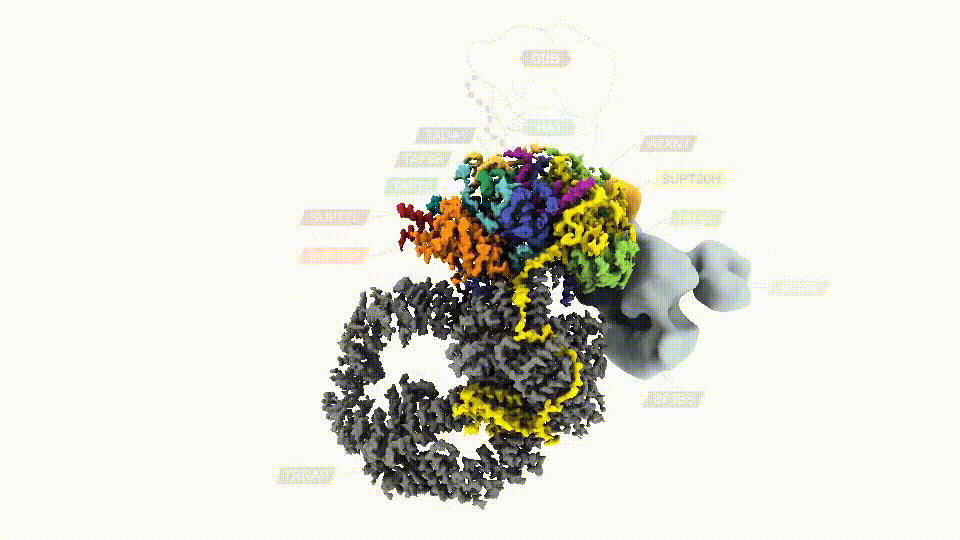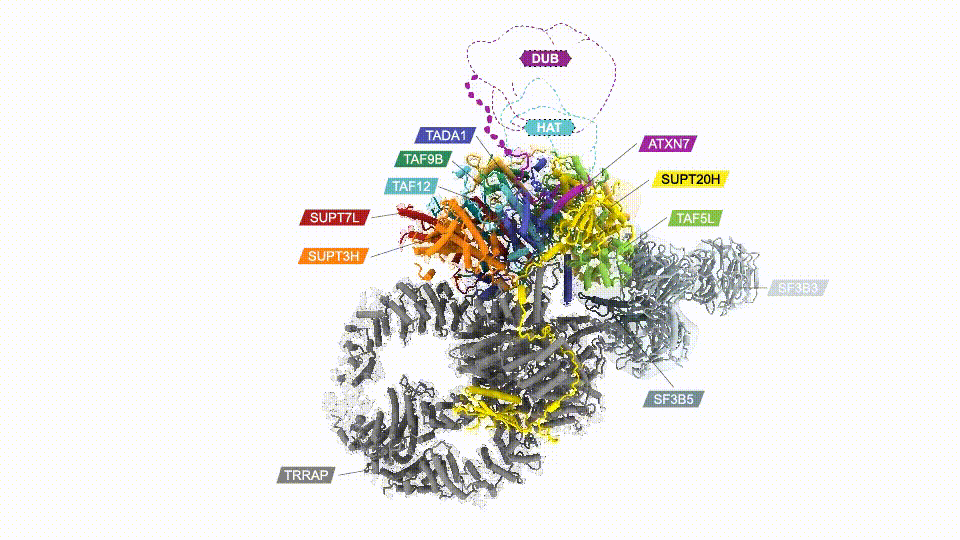
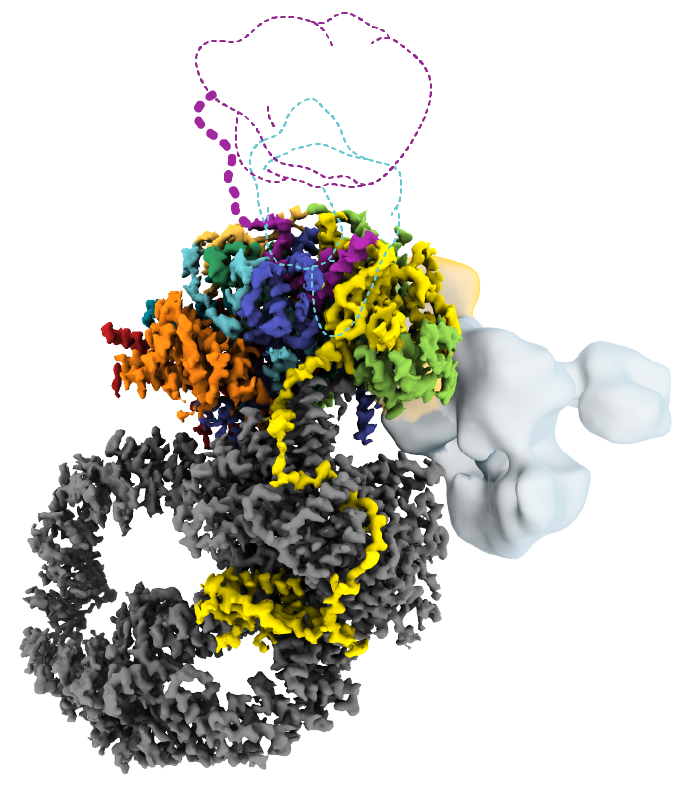
Researchers have, for the first time, mapped the human SAGA complex, a 20-part molecular machine that helps control gene activity. The colored sections represent SAGA’s core module. Two other major components are shown in gray. (Credit: D. Herbst et al./Nature Structural & Molecular Biology 2021)
Mapping SAGA’s structure—in yeast or humans—isn’t easy. In addition to its size, SAGA has flexible parts that make it difficult to visualize using conventional methods. Nogales’ team genetically modified SAGA with molecular handles so they could pull it out of lab-grown cells in sufficient quantities. Then they used cryo-electron microscopy (cryo-EM) to examine the complex. The final structure, reported in Nature Structural & Molecular Biology, revealed that two major modules of human SAGA are positioned in a different orientation relative to yeast. In addition, the researchers discovered a small component that acts as a latch securing one of SAGA’s mobile parts.
Nogales’ team also mapped the locations of the 13 known SAGA mutations linked to disease. With this physical guide, scientists can better examine the effects of these errors and potentially develop drugs to restore SAGA’s function.
Read more from HHMI.