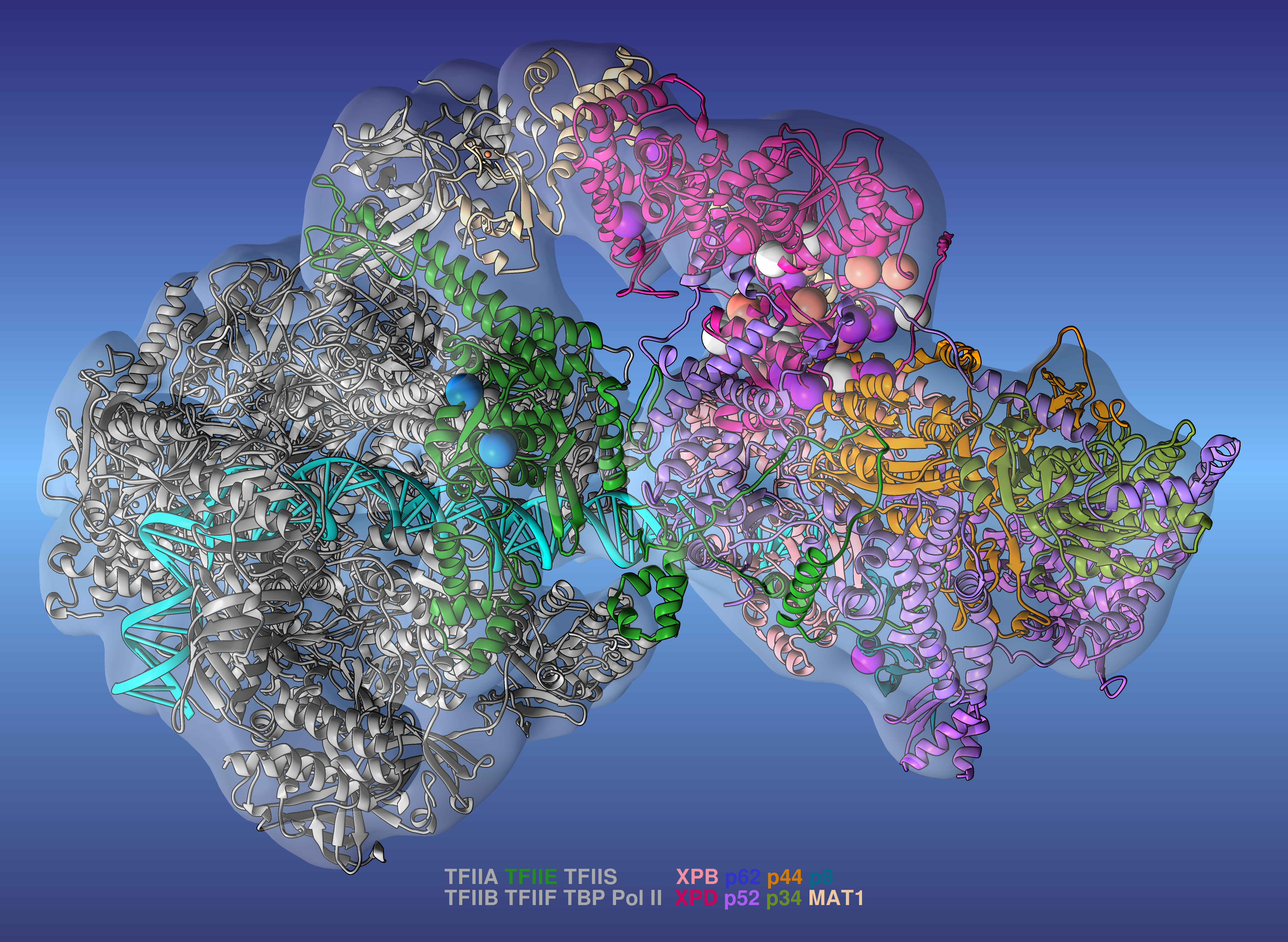
Researchers led by Ivaylo Ivanov of Georgia State University have produced a comprehensive model of the human transcription preinitiation complex (PIC), a vital assembly of proteins responsible for regulating gene expression. The new model is the most complete to date and provides mechanistic insights into how mutations affecting one component—human transcription initiation factor IIH, or TFIIH—lead to three inherited genetic diseases.
Berkeley Lab Biosciences’ Susan Tsutakawa, a research scientist in Molecular Biophysics and Integrated Bioimaging (MBIB), and John Tainer, a professor at the University of Texas MD Anderson Cancer Center and visiting faculty in MBIB, were part of the team. Results from this work were recently published in Nature Structural & Molecular Biology.
 To develop the PIC model, the researchers integrated all available data from cryo-electron microscopy (cryo-EM) to build a practically complete human PIC structural model. They then ran large-scale molecular dynamics simulations on the 200-petaflop Summit supercomputer at Oak Ridge National Laboratory. The simulations revealed the hierarchical organization of the PIC and explained how its numerous structural components function to modify DNA during the early stages of transcription. By mapping 36 different patient-derived mutations to the PIC model, the team determined that the mutations tend to cluster at crucial areas of TFIIH, interfering with its ability to unwind the double helix strands of DNA to initiate transcription.
To develop the PIC model, the researchers integrated all available data from cryo-electron microscopy (cryo-EM) to build a practically complete human PIC structural model. They then ran large-scale molecular dynamics simulations on the 200-petaflop Summit supercomputer at Oak Ridge National Laboratory. The simulations revealed the hierarchical organization of the PIC and explained how its numerous structural components function to modify DNA during the early stages of transcription. By mapping 36 different patient-derived mutations to the PIC model, the team determined that the mutations tend to cluster at crucial areas of TFIIH, interfering with its ability to unwind the double helix strands of DNA to initiate transcription.
Tsutakawa noted that the study used advanced computational methods to extend the cryo-EM work on TFIIH done by Eva Nogales, senior faculty scientist in MBIB. Tainer added that the breakthrough exemplifies the power of collaborations between Berkeley Lab and university researchers: The structures of the core TFIIH helicases responsible for unwinding DNA for transcription or repair were determined at the Advanced Light Source (ALS) synchrotron, and the expertise on TFIIH enabled cryo-EM data collection and interpretation of the human mutations centered on the helicases.
Read more from Georgia State University and Oak Ridge National Lab.