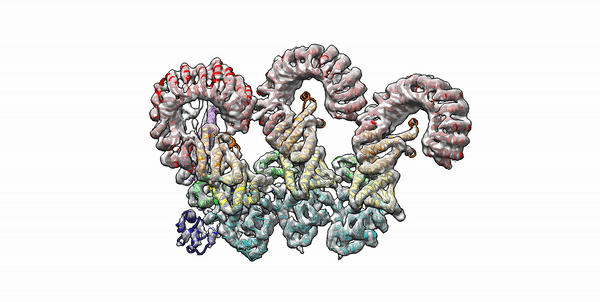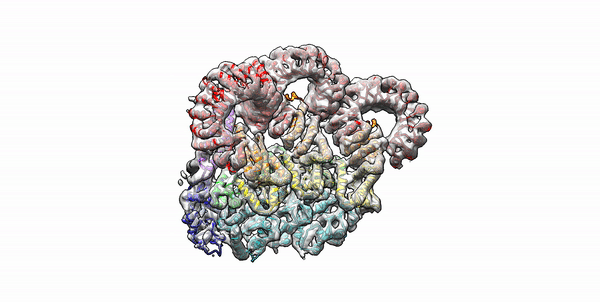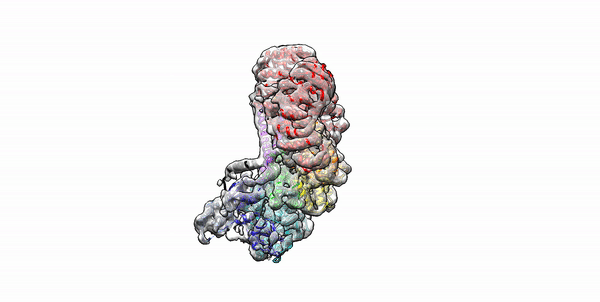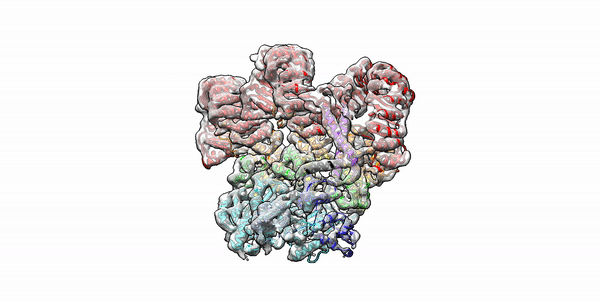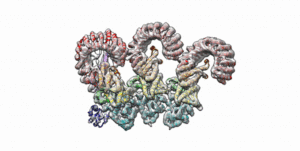
Cryo-EM structure of the first three subunits of an inflammasome, which consists of the NAIP5 and NLRC4 immune proteins. The NAIP5 subunit is bound to flagellin, shown in light purple. (Credit: Nicole Haloupek/UC Berkeley)
Berkeley Lab and UC Berkeley biologists used cryo-electron microscopy (cryo-EM) to resolve the structure of a protein complex, called an inflammasome, that is assembled within cells in response to incursion by a pathogen and functions as a beacon to summon immune system support. Inflammasome assembly is initiated when a protein called NAIP5 latches onto a flagellin molecule—a piece of the whiplike tail used by bacteria to propel themselves. Then several copies of another protein, NLRC4, join in to form the ring-shaped protein cluster. The researchers, led by Eva Nogales in Biosciences’ Molecular Biophysics and Integrated Bioimaging (MBIB) Division and Russell Vance in the Department of Molecular & Cell Biology at UCB, found that flagellin is in contact with six different parts of NAIP5. They further demonstrated that the multiple contact sites help prevent bacteria with minor mutations from eluding detection. The study was published in the journal Science. Read more in the Berkeley Lab News Center.