The use of X-ray Free Electron Lasers (XFEL) has yielded many exciting results in the study of biological systems and the physical process, such as experimental methods and data analysis techniques, continue to improve. Researchers from Lawrence Berkeley National Laboratory (Berkeley Lab) led a multi-institutional team that has published a game-changing method in Nature Methods that has been perfected at the Linear Coherent Light Source (LCLS) at SLAC National Acceleratory Laboratory in Stanford.
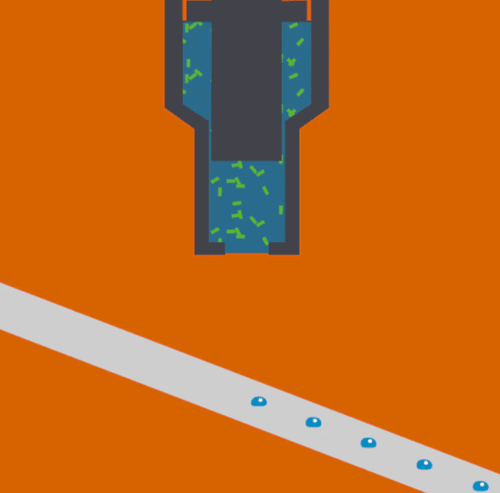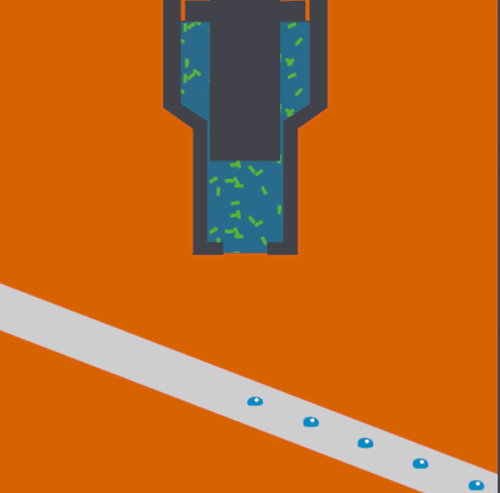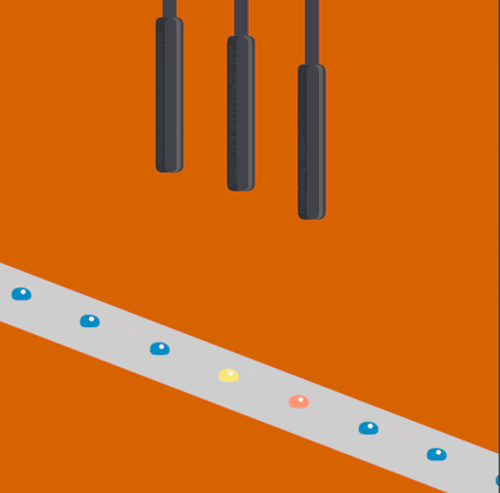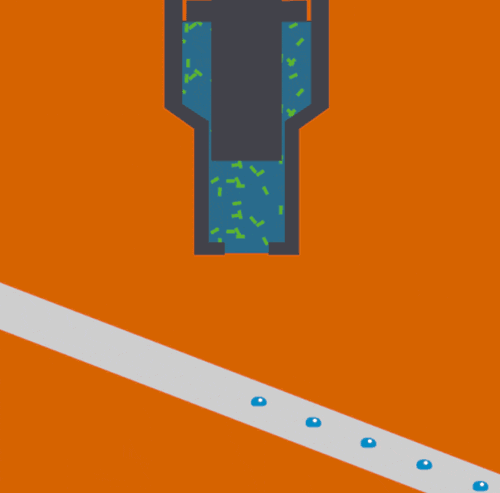
The XFEL beam is so powerful that experiments have been described as “diffract and destroy,” where the crystals containing biological molecules are obliterated within nanoseconds after initial exposure. Previously, most researchers used injectors to deliver a continuous stream of crystals to the beam, but this wastes a large portion of purified biological sample and limits the scope of systems that can be studied. The new method, dubbed the “Droplet on Tape” (DOT) method, exposes a single drop of the crystalline material to the beam at one time and allows for simultaneous physical analysis using other modes of detection (diffraction, spectroscopy, or scattering).
Acoustic droplet ejection (ADE), a method developed in collaboration by scientists at Berkeley Lab and Brookhaven National Laboratory, allows researchers to deposit tiny nanoliter drops of sample directly into the X-ray beam, considerably increasing the efficiency of sample consumption. Study senior author Junko Yano, senior scientist in the Molecular Biophysics and Integrated Bioimaging Division (MBIB) at Berkeley Lab, describes the current work as an extension of ADE technology, where instead of depositing droplets directly into the beam, they are instead placed on a conveyor belt. The team used ADE-DOT on a variety of enzymatic systems, demonstrating various modes of reaction initiation and real-time measurement.
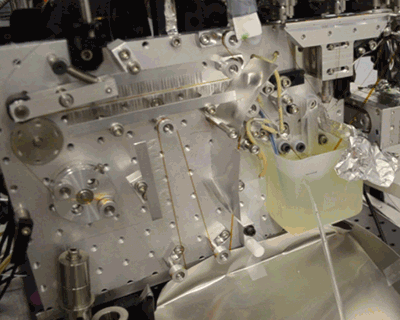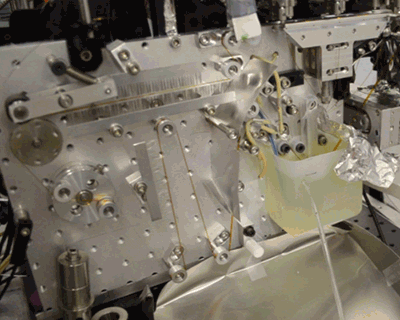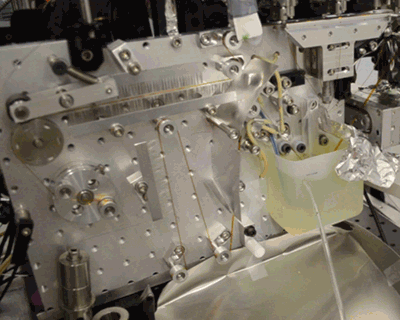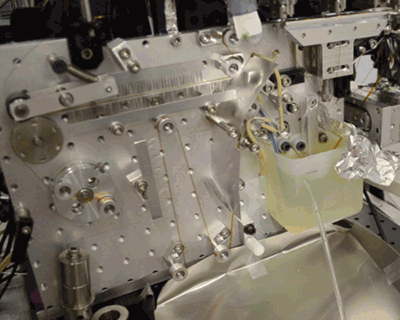
“The addition of the conveyor belt enables us to study both a wider and physiologically more relevant range of time scales, from microseconds to seconds, and a greater variety of ways to start transient reactions,” said Yano. “We wanted to use more than one technique at a time so that we can see what is happening on the atomic level inside the core of enzymatic molecules.” The investigators demonstrated both light-based and chemical reaction initiation, as well as multi-modal measurement capability with simultaneous x-ray crystallography and x-ray emission to give atomic structural and electronic information. Yano mentioned that this widens current capabilities, “For example, we can use ADE-DOT to investigate the actual electron rearrangements happening during a chemical reaction in large systems such as photosystem II.”
Co-first authors Franklin Fuller and Sheraz Gul, postdoctoral fellows in MBIB, highlighted another advantage of ADE-DOT, noting that XFELs can be used to study biological systems at room temperature. Fuller noted, “In traditional synchrotron studies, samples are normally frozen to avoid radiation damage. With XFEL, we are able to collect the data on femtosecond timescales before the laser beam destroys the samples, so there are no radiation damage artifacts.”
Similar to classical stopped-flow enzyme experiments, ADE-DOT can be used in the study of slower reactions (second timescales) by mixing liquids just before droplet ejection. For faster reactions, researchers can use a rapid mixing scheme either while they are creating the droplet or just after the droplet is delivered to the conveyor belt.
Yano said the researchers are excited by the experiments that ADE-DOT makes available and are already looking ahead to new applications. “We have shown that we can study biochemical reactions as they unfold in real time under less extreme conditions using an x-ray laser,” she said. “With this technology, the possibilities are endless.”
For more, read the SLAC announcement.


