More than half of all cancer patients receive some form of radiation therapy as part of their treatment. Advances in technology have enabled precision targeting of tumors, minimizing exposure of healthy tissues to radiation. Even so, acute and/or chronic injury to immune-system and blood-forming cells is a common side effect because these cells are exquisitely sensitive to radiation. This may lead to infection, bleeding, or anemia, which can interrupt treatment and result in inferior patient outcomes.
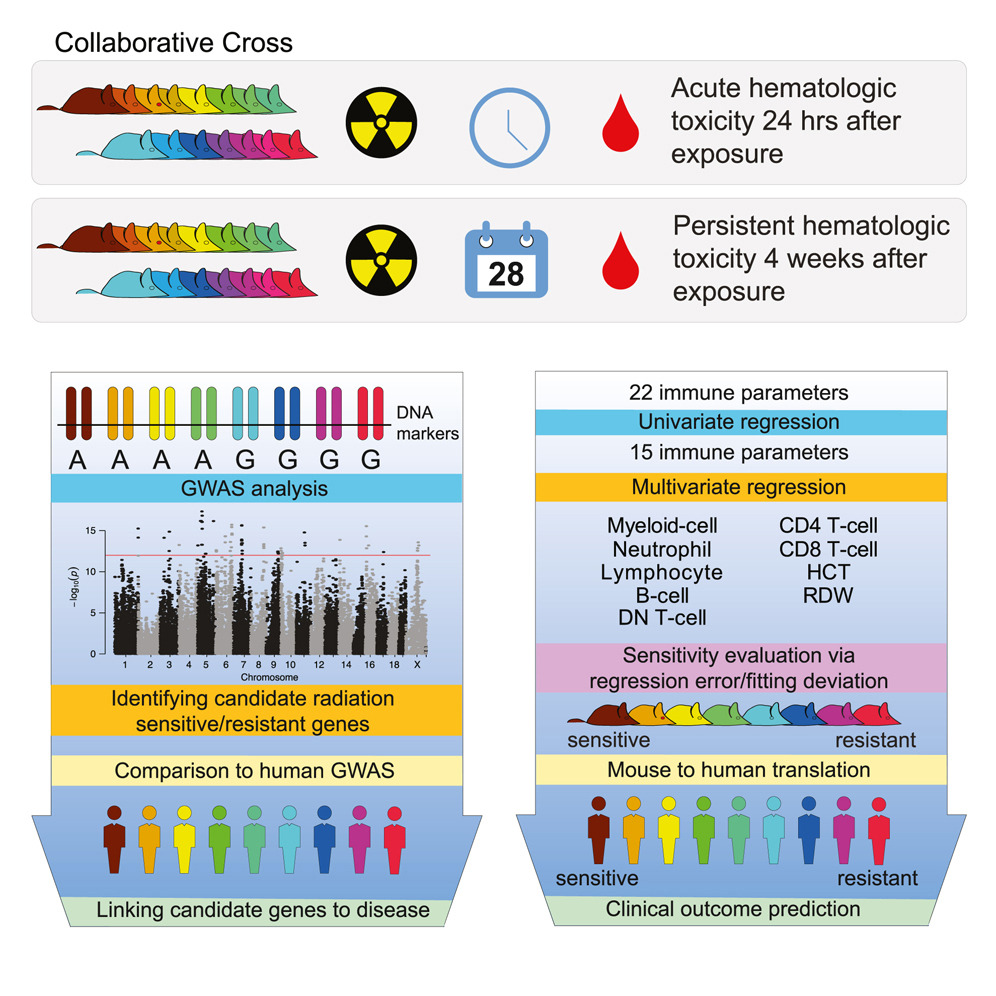
There appears to be a genetic component to the variability in how individuals respond to radiation exposure. Therefore, identifying biomarkers associated with radiation sensitivity/resistance would yield clues to the biological mechanisms underlying this variability—and may ultimately aid physicians in recommending optimal treatments for individual patients. Working toward these goals, researchers in the Biological Systems and Engineering (BSE) Division within the Biosciences Area at Berkeley Lab and their collaborators used a genetically diverse mouse population, the Collaborative Cross, to model radiation-induced injury to the hematopoietic system.
The researchers characterized radiation sensitivity by looking at the difference in a variety of immune parameters—for example, white blood cell count and hemoglobin (the oxygen-carrying molecule in blood) level—in irradiated mice compared to unirradiated controls. By doing this at two separate time points, they were able to derive sensitivity scores for both the immediate (24 hours) and persistent (4 weeks) response to radiation exposure and classify each genetically distinct mouse strain as either “sensitive,” “intermediate,” or “resistant” at each point.
“We found that they don’t necessarily agree very much; the early response does not really predict the long-term response to radiation,” said Antoine Snijders, a BSE senior scientist and Head of the BioEngineering & BioMedical Sciences Department, who was a corresponding author on the paper recently published in Cell Genomics. “It was interesting because we hypothesized that mice that were the most sensitive at 24 hours would have the hardest time recovering, but that wasn’t always the case.”
In fact, the variability in the responses to radiation exposure they observed in the mice closely mirrored that seen by physicians among human cancer patients. “Patients treated with toxic therapies—including radiation and chemotherapy—show marked variation in their recoveries following treatment,” noted Scott Kogan, an immunopathologist at UC San Francisco and contributing scientist. “The current work represents an important step towards being able to predict toxicity and to prevent deaths due to infection or hemorrhage.”
Because all of the single nucleotide polymorphism (SNP) gene variants are known for each mouse strain in the Collaborative Cross population, the researchers were able to perform genome-wide association studies (GWAS) to identify genetic loci associated with radiation sensitivity. About half of the candidate genes they identified in mice also turned up in human GWAS analyses of diseases or traits involving the immune system, suggesting that the mouse model can be used to study radiation sensitivity in humans.
“I think that the most important aim of this study is to try to find genetic factors to know which individuals are more vulnerable after radiation therapy, because they need special care,” said Jian-Hua Mao, a BSE senior scientist and corresponding author.
A serendipitous brush with Shannon MacDonald, a radiation oncologist at Massachusetts General Hospital and associate professor at Harvard Medical School, at a particle therapy conference led to a collaboration to translate the findings from the mice to humans. MacDonald and her colleagues Kevin Liu and Daphne Haas-Kogan at Brigham and Women’s Hospital had done a similar study of blood toxicity in children with medulloblastoma, a common type of brain tumor, treated with radiation therapy.
Radiation sensitivity scores for 80 of the human patients were derived based on a subset of immune parameters that overlapped with the mice and were determined via statistical analyses to be the most salient: total white blood cell count, neutrophil and lymphocyte (two types of white blood cell) counts, and red blood cell count. Interestingly, the patients classified as the most resistant—meaning their post-radiation blood cell counts changed the least compared to already low baseline levels—were ultimately more likely to have a cancer recurrence.
“This study provides evidence to support what we have suspected for decades: that patient-specific genetic factors contribute to their radiation sensitivity,” MacDonald said. “While it is difficult to improve upon the physical delivery of radiation, this represents a tangible way to improve outcomes for patients and underscores the importance of future research in this area.”
“The findings from this study are exciting and suggest that genetic factors from patients correlate with sensitivity to radiation. In the future, these findings may help inform the design of more personalized radiation treatment plans for patients,” Liu added.
The study authors have made all of the immune data from 1,800 mice across 35 different strains, plus the GWAS analyses, available to the broader research community to facilitate further investigation. “There’s a treasure trove of follow-up studies that could be done on this,” Snijders said.
Additional BSE-affiliated contributors to this work include: Li He, Jamie Inman, and Chenhan Zhong, who performed the mouse experiments; and Hang Chang, who led the computational design and model translation to human cancer patients and performed bioinformatics and statistical analyses. David Threadgill of Texas A&M University and Myrsini Ioakeim-Ioannidou of Massachusetts General Hospital also contributed.




