Gently turning the knobs on a control panel, Agustin Avila Sakar is fine-tuning an experimental setup via a computer interface. He’s preparing an electron microscope for a several hours-long session that will automatically record thousands of images. Later on, this mosaic will help researchers determine the high-resolution structure of proteins. With regards to structure, proteins may be the most diverse class of molecules known, and mapping their shape can serve as a blueprint for function.
“Structural information enriches our knowledge of cells and how they interact with the environment,” said Sakar, the cryo-EM manager for Berkeley Lab.
To reveal these intricate, enigmatic structures, Sakar and his team use cryogenic electron microscopy, or cryo-EM for short. This technique passes electrons through a sample held at a very low temperature. Based on how the electrons move through the material and get scattered by its atoms, scientists can determine the structure and infer function of the macromolecule.

This form of electron microscopy was invented because the high energy electrons moving through softer samples, like proteins in cell organelles, damage the very molecules that are to be imaged. “Then you don’t see the molecules in their native form, instead they’re transformed by the action of the electron beam,” Sakar explained. “So you have to devise means of minimizing that effect.”
Resolution Revolution
One way to avoid this is to snap a photo of the sample before the electrons jumble up the molecular structure. To do this, Sakar said, “We have to slow everything down.” In the first step, the flash-freeze, they lower the temperature of the sample to -170 degrees Celsius in a fraction of a millisecond and then hold it at -180 degrees Celsius while the imaging happens.
Sakar has spent the last three decades studying the techniques and tools needed to execute this precise way of revealing macromolecular structure. Cryo-EM techniques first came onto the structural imaging scene in the 1970s and gained significant momentum in the ‘80s. Sakar recalls being introduced to the growing field during his studies at Baylor College of Medicine in Texas.

“Until this invention, electron microscopy in biological sciences was limited,” he said. Images of living samples, like organelles and macromolecules in cells, wouldn’t generate an accurate representation of the underlying molecular structure. Cryo-EM, on the other hand, enabled glimpses into the native structures—and opened up new worlds of opportunity for discovery and technological advancement. “I really saw that there was a path and a challenge to push towards solving structures at the highest resolution possible.”
After completing his doctorate in molecular physiology and biophysics and a postdoctoral position under the late Ken Downing at Berkeley Lab, Sakar headed towards the industry side of cryo-EM. He spent time at FEI Company (now part of Thermo Fisher Scientific) and Gatan (now part of Ametek), both powerhouse contributors to the development and advancement of electron microscopes and electron detectors for cryo-EM, respectively. “We were able to show atomic resolution structures by cryo-EM in just a few weeks, when it previously would’ve taken years,” Sakar said. “This in the scientific community is now referred to as the Resolution Revolution.”
Back to the Research
After years spent optimizing the technology of cryo-EM, Sakar had “a yearning to come back to the research.” In October 2021, he rejoined Berkeley Lab to plan and manage the new cryo-EM facility slated to be located in the state-of-the-art Biological and Environmental Program Integration Center (BioEPIC).
Scheduled to be completed in 2024, BioEPIC is currently under construction. This 73,000 square foot facility will house Berkeley Lab employees from both the Biosciences Area and the Earth and Environmental Science Area, with four DOE-funded Scientific Focus Area anchor tenants coming together under one roof: Belowground Biogeochemistry, ENIGMA, m-CAFEs, and Watershed Function.
For Sakar, overseeing the cryo-EM facility and offering its resources to these groups presents exciting challenges. Not only will he be tasked with understanding the goals of each team’s area of research, he’ll anticipate advancements in the cryo-EM capabilities to make calculated updates to the instrumentation, data recording, and processing protocols, as well as suggestions to further their future studies.

A current trend that is growing in popularity, according to Sakar, is correlative microscopy. This is when one sample is passed through multiple types of electron microscopes, cryo-EM included, to yield an in-depth, multidimensional view of macromolecular structure. “That enriches the information tremendously,” he said.
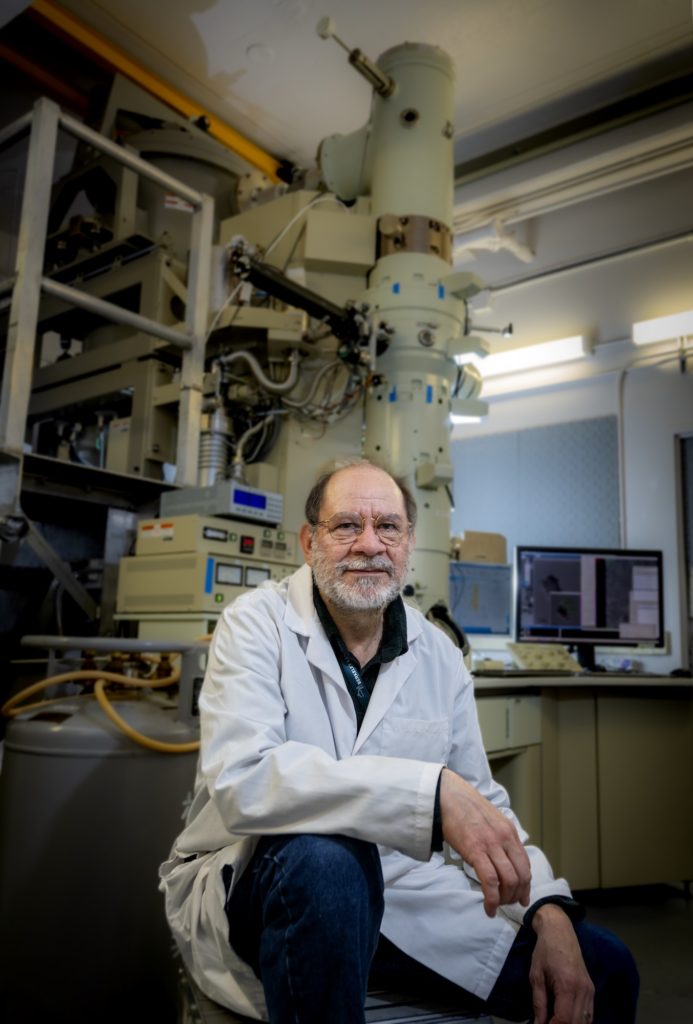
In addition to forging new partnerships across the BioEPIC groups, Sakar is tasked with designing the space that will house the cryo-EM instruments. With such deep experience in this technology, he’s able to incorporate the immediate needs of current equipment—and anticipate, as best as possible, the advancements that seem likely to unfold in the near future.
“I feel confident because I’m familiar enough with the equipment and the Lab,” Sakar said. “I can propose ideas and I can learn from others. I am excited by the complexity of science.” ⬢
Written by Ashleigh Papp, a communications specialist for Berkeley Lab’s Biosciences Area.
Read other profiles in the Behind the Breakthroughs series.




