Scientists who specialize in studying the atom-by-atom choreography of enzymes have revealed new insights into the function of isopenicillin N synthase, an enzyme needed to produce some of the world’s most critical antibiotics. Their study was published in Science Advances.
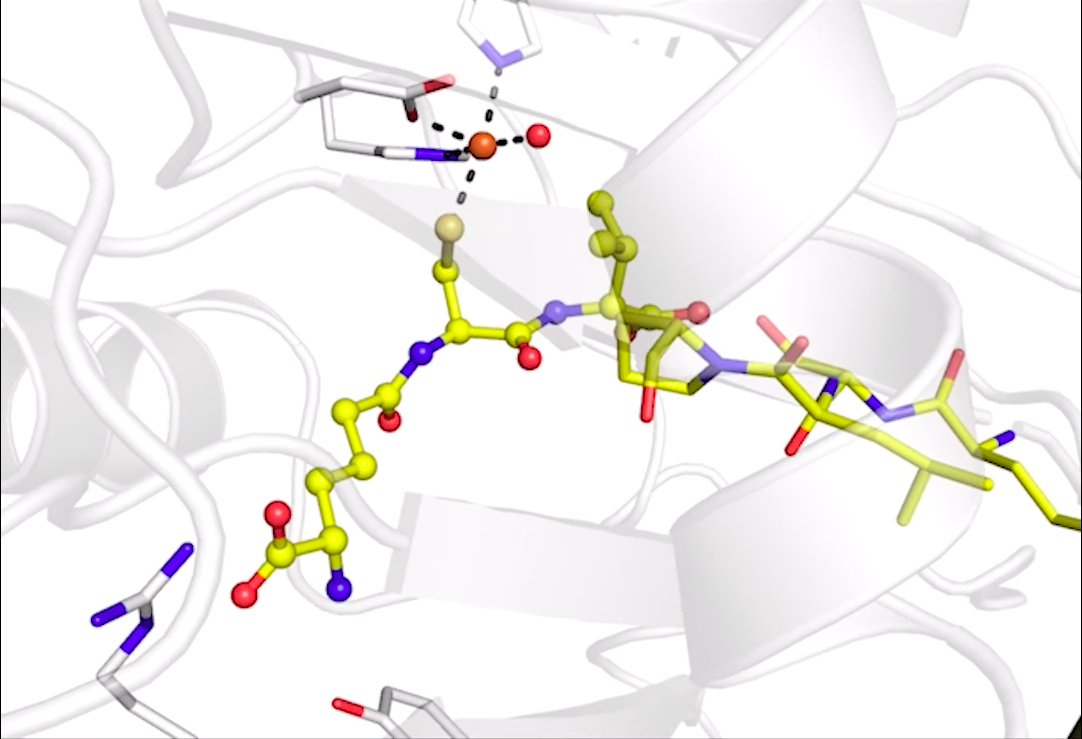
The team of scientists from Oxford University, Diamond Light Source, and Berkeley Lab used a combination of X-ray methods to generate an action storyboard showing how isopenicillin N synthase remodels a linear precursor molecule into a distinctive double-ringed molecule. This two-ringed molecule is the starting structural template for a large class of complex molecules with antibiotic properties known as beta-lactams.
“Penicillin is one of the first antibiotics discovered and is still widely used, but the biological process that is responsible for the formation of penicillin and related cephalosporin antibiotics is not completely understood,” said Jan Kern, a Berkeley Lab staff scientist and one of the new study’s lead authors. “We used our ‘molecular movie’ approach to take snapshots of the enzyme while it is reacting with its substrate and molecular oxygen, and we are now able to better understand the first steps in this ring-closure reaction.”
According to Kern, the new details will help scientists modify isopenicillin N synthase to synthesize other novel antibiotics and also have implications for understanding the reaction mechanism of a larger group of related enzymes, including those involved in DNA/RNA repair and the human tissue response to low oxygen.
The team’s approach combines X-ray crystallographic and X-ray spectroscopic data to examine the movement of specific atoms at different time points in a reaction. In the case of isopenicillin N synthase, the scientists studied how an iron atom in the enzyme’s active site interacts with molecular oxygen (O2) to catalyze the ring-building reaction. The data was gathered at Diamond Light Source, in the United Kingdom; the Sub Angstrom Compact Light Source in Japan, and the Linac Coherent Light Source (LCLS) at SLAC National Accelerator Laboratory.
This Science Snapshot appeared in the Berkeley Lab News Center.