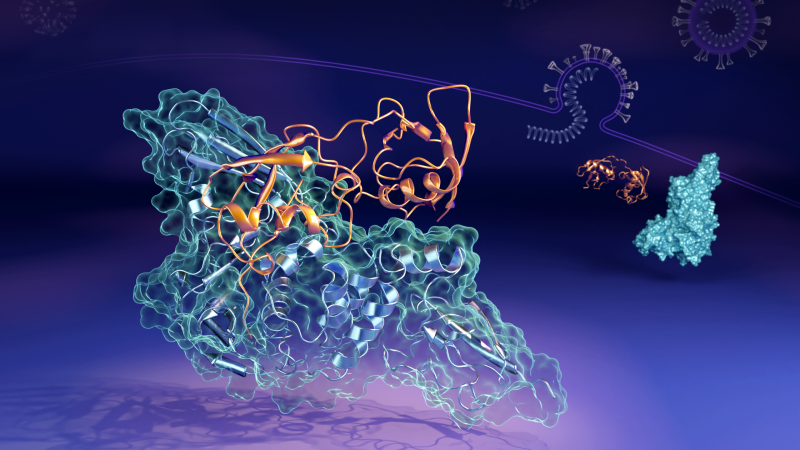
ORNL and LBNL researchers found the papain-like protease (in orange) can bind to the human interferon-stimulated gene 15 protein (in blue) in multiple ways and shapes. (Credit: ORNL/Jill Hemman)
Papain-like protease (PLpro) from SARS-CoV-2 plays essential roles in the replication cycle of the virus that is the cause of the global COVID-19 pandemic. In human cells that the virus has infected, PLpro seeks out and binds with the interferon-stimulated gene 15 (ISG15) protein, a key component of the cells’ immune response. PLpro strips ISG15 from other cellular proteins to aid SARS-CoV-2 in evading the body’s immune system.
Scientists at Oak Ridge National Laboratory (ORNL) used small-angle neutron scattering (SANS) at the High Flux Isotope Reactor (HFIR) combined with computational techniques to reveal the molecular details of how the two proteins interact. Susan Tsutakawa, a staff scientist in the Molecular Biophysics and Integrated Bioimaging (MBIB) Division, obtained small-angle x-ray scattering (SAXS) data on the PLpro-ISG15 complex at Berkeley Lab’s Advanced Light Source (ALS) to augment the SANS work.
“In the SAXS studies, we could separate out different complexes in the sample by coupling SAXS with size-exclusion chromatography and, at the same time, get higher-resolution data of the complex’s overall configuration to complement the SANS studies that revealed the conformations of individual components in the complex,” Tsutakawa said.
As they reported in the Journal of Physical Chemistry Letters, the scientists showed that human ISG15 undergoes a transition from an extended to a compact state after binding to PLpro from the SARS-CoV-2 virus. Such a conformation had not been previously observed in complexes of SARS-CoV-2 PLpro with ISG15 from other species, such as bats, and may contribute to inter-species differences in immune response.
The team plans to conduct additional experiments on this type of biological complex to examine how small molecules can block the binding of PLpro to ISG15 and allow a person’s immune system to better fight the invading virus.
Read more from ORNL Neutron Sciences.