Chemotherapy and radiation treatments can be life-saving for patients with cancer, but they have harsh side effects that can been felt and seen throughout the body. There can also be unseen consequences: These important treatments can mutate DNA and damage chromosomes in patients’ cancerous and noncancerous cells alike. When this occurs in a germline cell (eggs in women and sperm in men), it can lead to serious fetal and birth defects in a resulting pregnancy.
For the few chemotherapies that have been studied, the risk of mutated sperm diminishes over time, as the treatment agents leave the body and men produce new sperm that were never exposed. But there is currently no efficient and affordable test that can be used to track when sperm are carrying treatment-related chromosomal mutations such as aneuploidy (abnormal number of chromosomes) or chromosome breaks, rearrangements, or deletions.
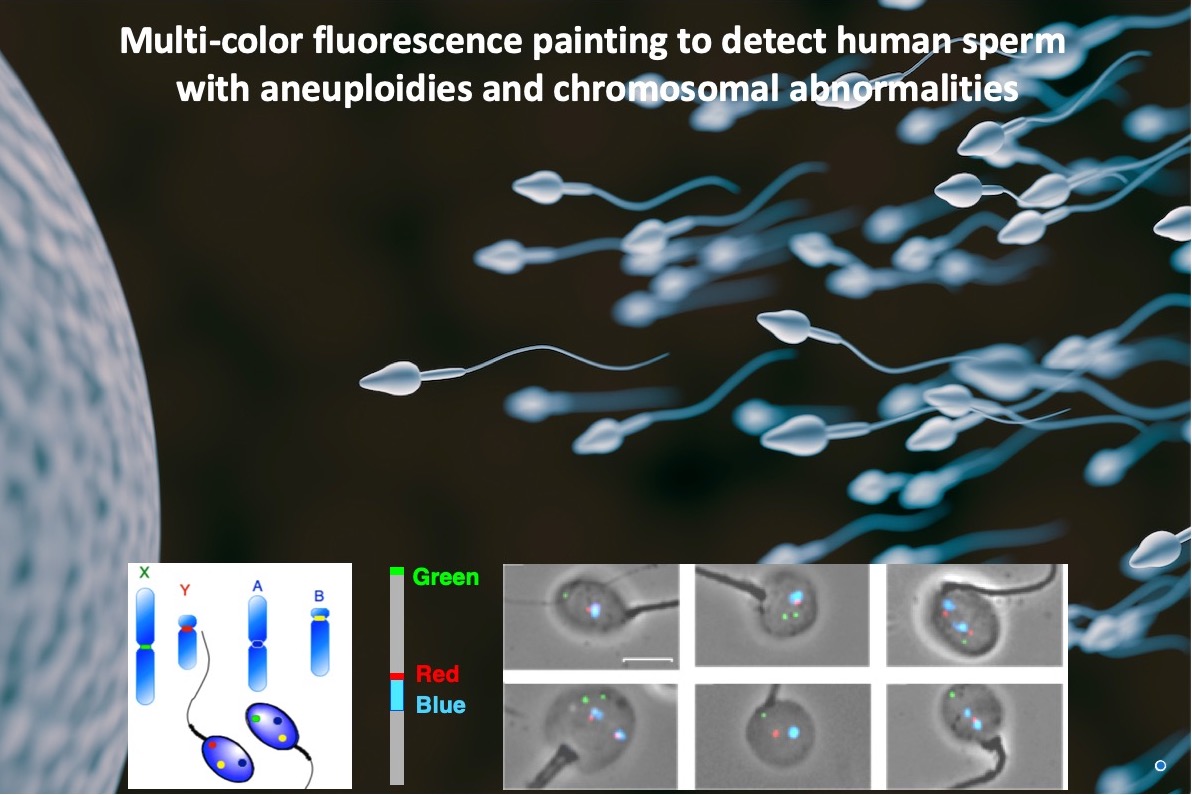
 In a study published in PLOS One, a team led by Biological Systems and Engineering (BSE) Division senior scientist Andrew Wyrobek reported success adapting an established cellular DNA analysis technique called fluorescence in situ hybridization (FISH) to probe sperm DNA for a wide variety of chromosomal defects simultaneously. This version of the FISH technique, known as the AM8 sperm FISH protocol, is the result of decades of work done by Wyrobek’s research team.
In a study published in PLOS One, a team led by Biological Systems and Engineering (BSE) Division senior scientist Andrew Wyrobek reported success adapting an established cellular DNA analysis technique called fluorescence in situ hybridization (FISH) to probe sperm DNA for a wide variety of chromosomal defects simultaneously. This version of the FISH technique, known as the AM8 sperm FISH protocol, is the result of decades of work done by Wyrobek’s research team.
The assay can detect balanced chromosomal abnormalities, which are rearrangement with no loss or gain of genetic material. Balanced rearrangements are compatible with live birth and heritable to future pregnancies; affected children are likely to experience reduced fertility when they want to have children of their own.
The team—which included scientists from Lawrence Livermore National Laboratory, Stanford University, MD Anderson Cancer Center, and the National Autonomous University of Mexico—evaluated the AM8 FISH approach on sperm from nine Hodgkin lymphoma patients, who provided samples before, during, and after a multi-drug treatment regimen and radiation therapy. The results indicated that sperm produced during the cancer treatment had 10 times more chromosomal defects compared with sperm produced prior to treatment. But by month six post-treatment, the patients’ sperm had returned to pre-treatment quality.
“We are excited by these results because they are a first step toward applying this method to any human situation— such as aging, illness, drugs, or exposure to environmental toxicants—to determine genetic risks to male germ cells and to examine the persistence of chromosomally damaged sperm,” said Wyrobek. “We believe this approach has a wide range of applications in healthcare and family planning, as it can be used to identify environmental exposures that increase the risk for producing chromosomally abnormal sperm that can affect the health of future pregnancies and children for generations to come.”
Read more in the Berkeley Lab News Center.