Form I rubisco, an enzyme found in plants, algae, and cyanobacteria, has a deep evolutionary history going back nearly 2.4 billion years to the Great Oxygenation Event, when cyanobacteria transformed the Earth’s atmosphere by introducing oxygen through photosynthesis. Rubisco’s role in this foundational event makes it a key focus of scientists studying the evolution of life, as well as scientists seeking to develop bio-based fuels and renewable energy technologies.
Form I rubisco, an enzyme found in plants, algae, and cyanobacteria, has a deep evolutionary history going back nearly 2.4 billion years to the Great Oxygenation Event, when cyanobacteria transformed the Earth’s atmosphere by introducing oxygen through photosynthesis. Rubisco’s role in this foundational event makes it a key focus of scientists studying the evolution of life, as well as scientists seeking to develop bio-based fuels and renewable energy technologies.
In a study appearing in Nature Plants, researchers from UC Davis, UC Berkeley, and Berkeley Lab report the discovery and characterization of a previously undescribed lineage of form I rubisco – one that the researchers suspect diverged from form I rubisco prior to the evolution of cyanobacteria. The novel lineage, called form I’ rubisco, gives researchers new insights into the structural evolution of form I rubisco, potentially providing clues as to how this enzyme changed the planet.
The work was led by Patrick Shih, a UC Davis assistant professor and the director of Plant Biosystems Design at the Joint BioEnergy Institute (JBEI), and Doug Banda, a postdoctoral scholar in his lab.
Study co-author and collaborator Jill Banfield, of UC Berkeley’s Earth and Planetary Sciences Department, uncovered form I’ rubisco after performing metagenomic analyses on groundwater samples. Metagenomic analyses allow researchers to examine genes and genetic sequences from uncultured microorganisms found in the environment.
Using the genes and genetic sequences provided by Banfield, who is also a Berkeley Lab faculty scientist with a secondary appointment in Biosciences’ Environmental Genomics and Systems Biology (EGSB) Division, Banda and Shih successfully expressed form I’ rubisco using E. coli.
To learn how this newly identified form functions and how it compares to previously discovered rubisco enzymes, the scientists needed to build precise, 3D models of its structure. For this task, the lead authors turned to Paul Adams, Henrique Pereira, and Michal Hammel in the Molecular Biophysics and Integrated Bioimaging (MBIB) Division.
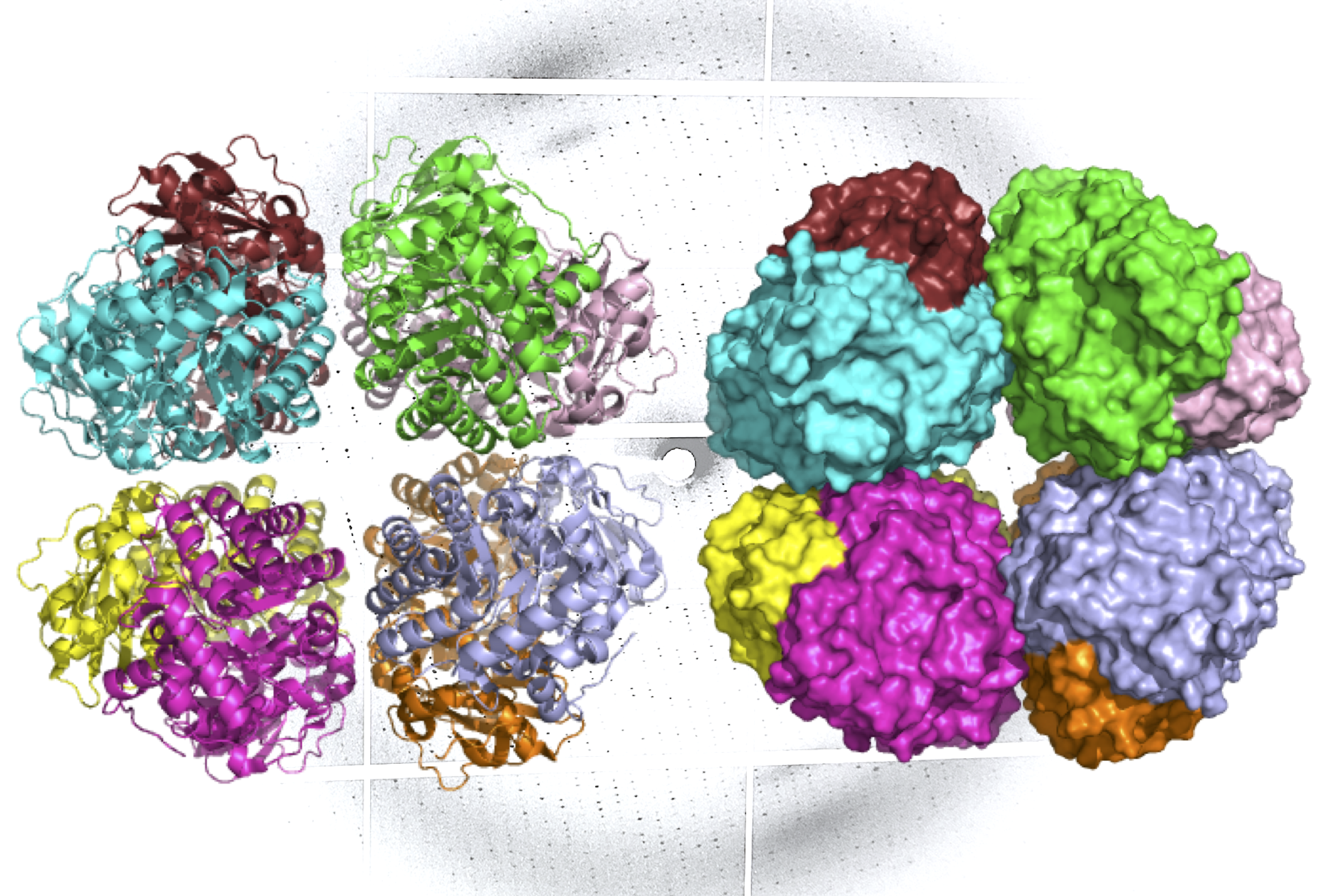
A ribbon diagram (L) and molecular surface representation (R) of carbon-fixing form I’ rubisco, showing eight molecular subunits without the small subunits. An X-ray diffraction pattern of the enzyme, also generated by the research team, is in the background. (Credit: Henrique Pereira/Berkeley Lab)
First, Adams and Pereira performed X-ray crystallography – an approach that can generate images of molecules with atomic-level resolution – at Berkeley Lab’s Advanced Light Source (ALS). Then, to capture how the enzyme’s structure changes during different states of activity, Hammel applied a technique called small-angle X-ray scattering (SAXS) using the SIBYLS beamline at the ALS.
The ALS investigations showed that like form I rubisco, form I’ rubisco is built from eight large subunits. However, it doesn’t possess the small subunits that were previously thought to be essential to its carbon-fixing function. The researchers now believe that form I’ rubisco represents a missing link in the evolutionary history of form I rubisco’s structure.
Read more in the Berkeley Lab News Center.