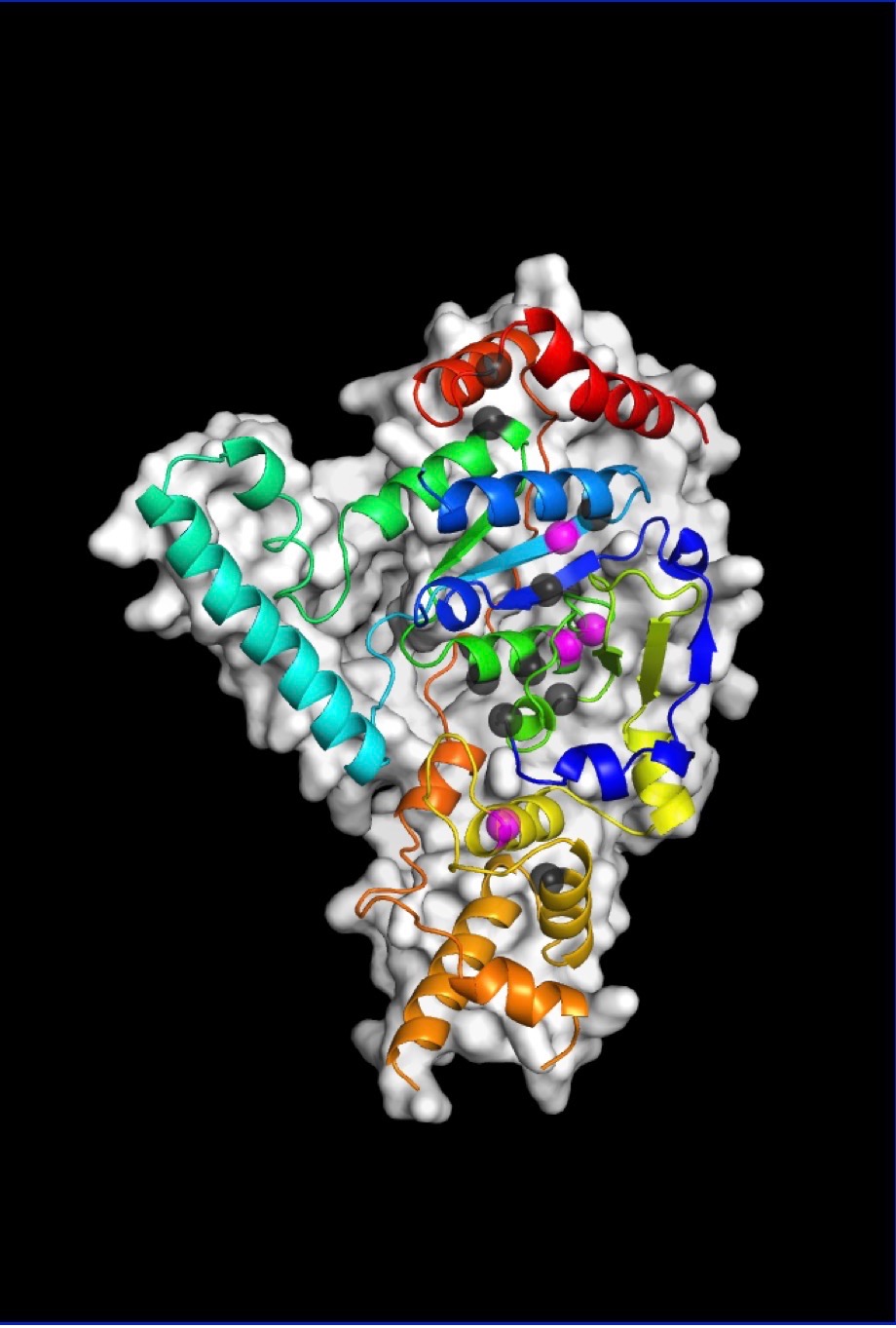
Biosciences Area researchers and their collaborators have determined how a protein called XPG binds to and reshapes damaged DNA, illuminating its role in averting genetic disease and cancer.
Susan Tsutakawa, a research scientist in Molecular Biophysics and Integrated Bioimaging (MBIB), Priscilla Cooper, a senior scientist in Biological Systems and Engineering (BSE), and John Tainer, visiting faculty in MBIB and director of structural biology at the University of Texas MD Anderson Cancer Center have been collaborating for 20 years to study the DNA-cleaving enzyme XPG.
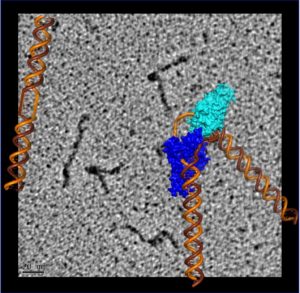
Discontinuous DNA strand bent by a bound XPG catalytic core overlaid on an electron micrograph of full-length XPG protein bound to a central bubble of discontinuous DNA. (Credit: Jack Griffith/UNC Chapel Hill and Susan Tsutakawa/Berkeley Lab)
Now, using a trifecta of cutting-edge imaging technology, the team was finally able to build a precise model of XPG’s catalytic core (the region responsible for the DNA cutting activity) and produce images of the large, multiple-unit molecule’s overall structure. An unexpected finding was that the flexible parts of the protein, which were previously impossible to examine, have the ability to recognize perturbations associated with many different types of DNA damage
They performed X-ray crystallography at Stanford Synchrotron Radiation Laboratory, small angle X-ray scattering (SAXS) at the SIBYLS beamline of Berkeley Lab’s Advanced Light Source, and rotary shadowing electron microscopy at the Lineberger Comprehensive Cancer Center at UNC Chapel Hill.
SAXS is a technique that allows scientists to analyze flexible molecules moving freely between their natural states rather than in static conformations, as necessitated by crystallography. Rotary shadowing electron microscopy allows direct visualization of individual DNA molecules with proteins bound to them. And crystallography performed on the catalytic core shed light on how inherited patient mutations in the gene for XPG can translate into severe protein dysfunction and different diseases.
The study was published in PNAS.
Read more in the Berkeley Lab News Center.