Metalloenzymes play an important role in biological systems, including physiology, nitrogen fixation, and photosynthesis. Understanding the fundamental chemical mechanism of enzymes is critical for optimizing the biochemical pathways for many aspects of life.
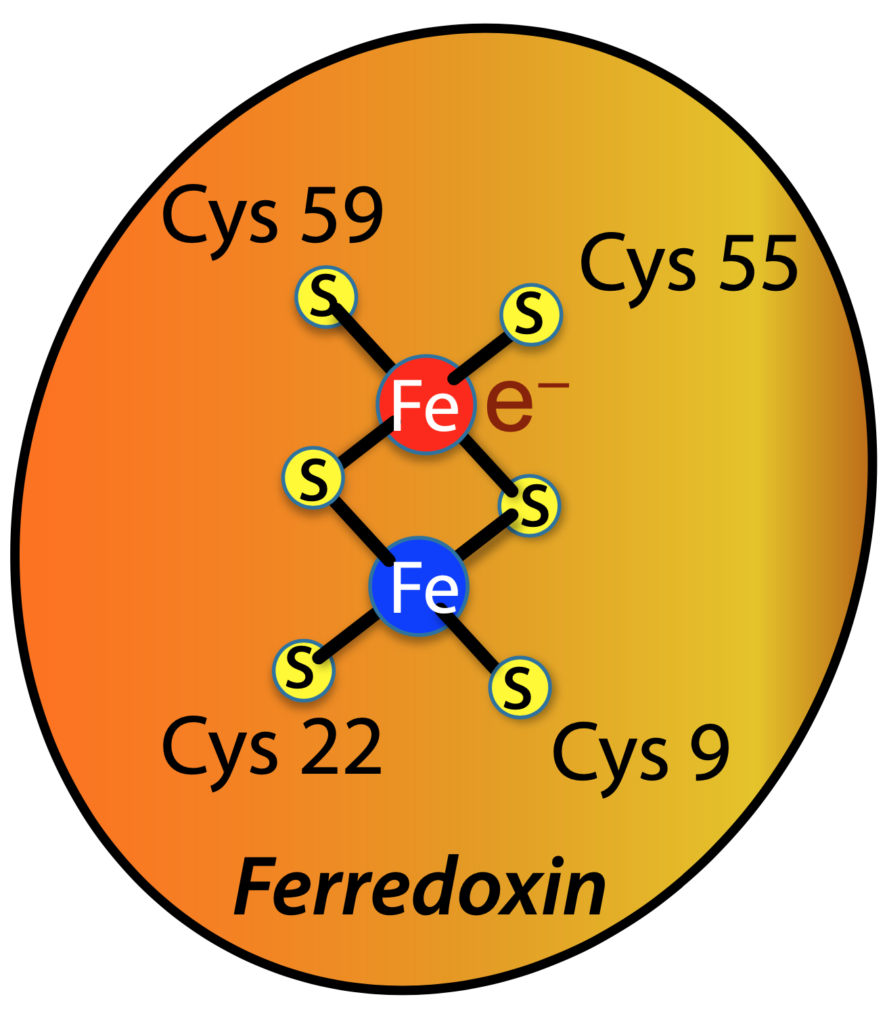
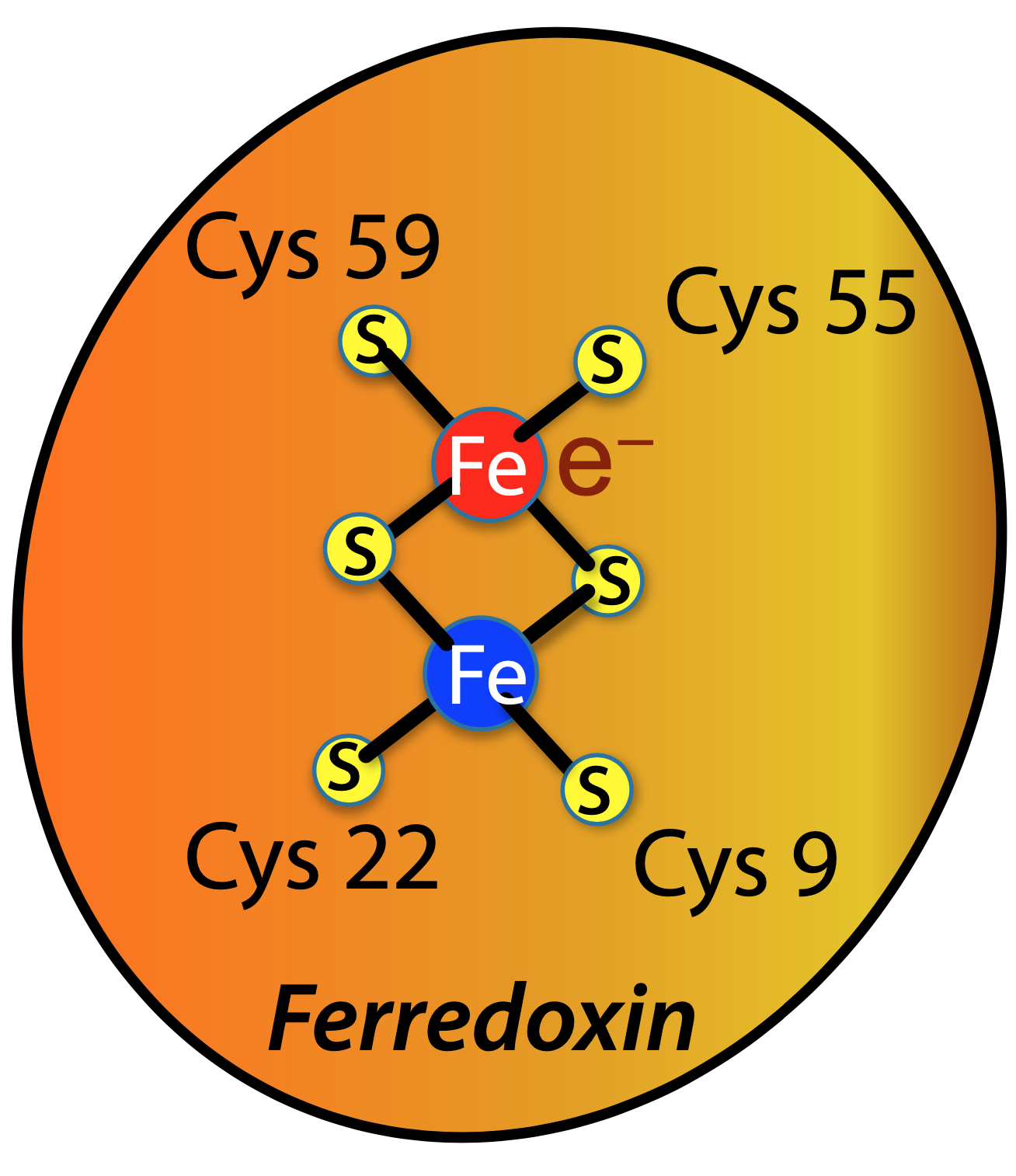
In photosynthesis, two iron atoms (Fe) in the protein ferredoxin shuttle an electron (e—) from one part of the photosynthetic machinery to another. The new paper is focused on determining the location of the electron, carried either by one iron atom or the other, by measuring its effect on X-ray diffraction.
It is well known that X-ray diffraction allows a crystallographer to examine the position of each atom in a protein structure. However, key enzymes containing active site metals–like photosystem II, which plays an integral role in photosynthesis–often function by moving electrons from one place to another. This is very hard to detect using X-rays, since these reactions produce just a 1% difference in the diffraction pattern.
In a paper published in Acta Crystallographica Section D, scientists in the Molecular Biophysics and Integrated Bioimaging (MBIB) Division present new computational methods that will enable metalloprotein studies at X-ray Free Electron Laser (XFEL) light sources. These XFELs (like the LINAC Coherent Light Source at the SLAC National Accelerator Laboratory) are special because proteins can be examined at room temperature under their functional conditions and can show how chemical reactions progress in real time.
Nicholas Sauter, MBIB senior scientist and first author of the study, explained that the goal of the team, and indeed many researchers who study metalloenzymes, is to see the electron changes in the metals of the proteins using XFELs. “We want to experimentally demonstrate that we can follow the progress of an oxidation/reduction (or redox) reaction by XFEL crystallography,” said Sauter. This paper lays the groundwork for those experiments, detailing the researchers’ new computational methods to achieve the visualization of redox reactions.
“While we hope to realize actual experiments shortly,” explained coauthor James Holton, “what is particularly exciting in this moment is that we show there is still untapped potential in the 106-year old technique of X-ray crystallography.” Holton is an MBIB faculty scientist, and is also an adjunct professor at the University of California, San Francisco.
Traditionally, metal sites are examined using spectroscopy, but X-ray diffraction offers the unique ability to spatially resolve multiple metal sites for proteins having more than one metal atom. Therefore, with this work, Sauter and his co-authors propose that X-ray crystallography can be utilized as a spectroscopic technique. “In other words, while the standard use of crystallography is to locate atomic positions, we can also locate electrons in the structure if we measure very carefully and do a lot of calculations,” Sauter said.
Those calculations were performed using the supercomputing resources of the National Energy Research Scientific Computing Center, a DOE Office of Science User Facility.
Other co-authors of this paper were Jan Kern and Junko Yano, both of the MBIB Division.