Scientists have developed myriad new tools to probe the microscopic world inside cells, but each comes with a trade-off. Light microscopy makes identifying specific cellular structures simple by tagging them with easy-to-see fluorescent molecules. But fluorescence can reveal only a handful proteins (out more than 10,000) in a cell at a given time, making it difficult to understand how they relate to everything else. Electron microscopy reveals all cellular structures in high-resolution, but delineating one feature of interest from the rest is difficult because the space inside of cells is so crowded.
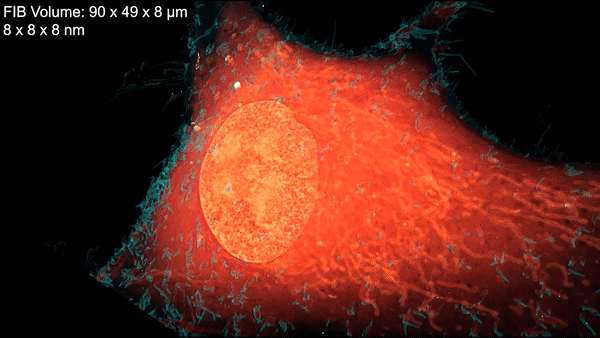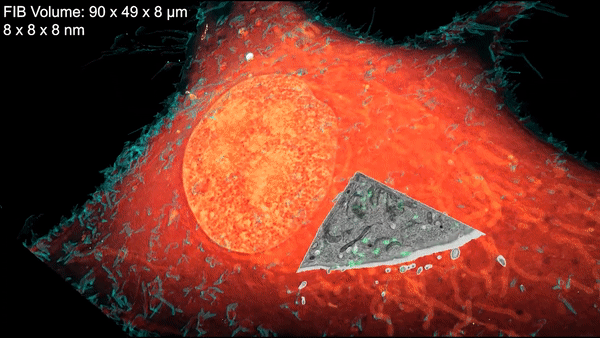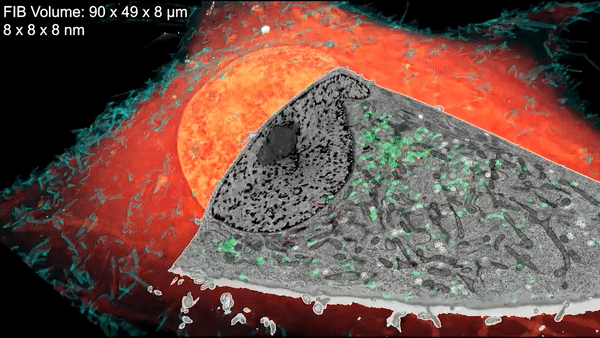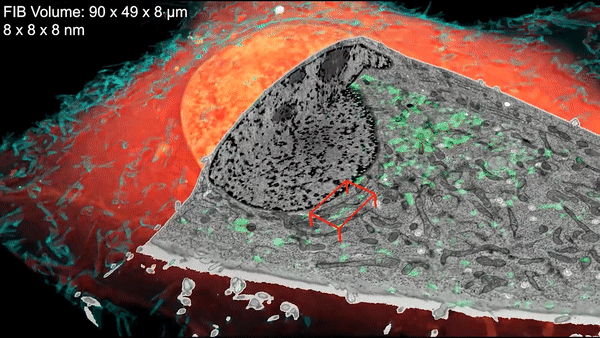
A new microscopy technique combines electron microscopy and light microscopy to generate detailed, three-dimensional images of cells. Credit: D. Hoffman et al./Science 2020
Now a team led by Harald Hess at the Howard Hughes Medical Institute (HHMI) Janelia Research Campus and Eric Betzig, a senior faculty scientist in the Molecular Biophysics and Integrated Bioimaging (MBIB) Division, has devised a technique that combines cryogenic super-resolution fluorescence microscopy and focused ion beam–milling scanning electron microscopy. In a report in the journal Science, the researchers describe their technique, called cryo-SR/EM, and display some of exquisitely detailed three-dimensional images they captured of the complex innards of cells.
“Bringing two very different imaging modalities together … brings insights into cellular structure at the nanoscale, which cannot be obtained by either alone,” said Betzig, who is also a UC Berkeley professor of molecular and cell biology and of physics and a HHMI investigator.
Read more from the UC Berkeley News Center.



